Prof. Nam's goal: a 'new integrative paradigm' of hearing research
Thirty-seven years ago, English researcher David Kemp discovered that the human inner ear not only receives but also generates sounds as part of its normal functioning. This finding led to the standard method now used to screen hearing in newborns.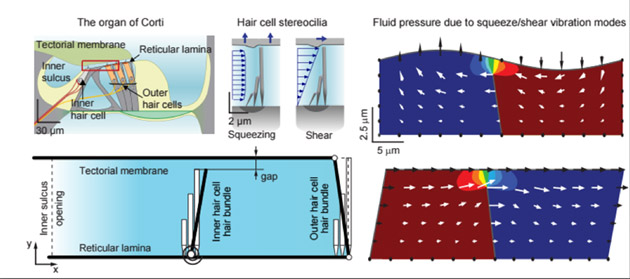
A cross section of the organ of Corti at upper left shows the proximity of outer and inner hair cells to the tectorial and basilar membranes. The other diagrams show hair cells modulating power dissipation within the inner ear for optimal amplification and tuning of sounds. Asst. Prof. Jong-Hoon Nam hopes to clarify how the dissipation of this energy – an “underappreciated but crucial aspect”— occurs, to illustrate the overall balancing act that occurs within the cochlea.
And yet, even now, scientists are not sure how or why these “otoacoustic emissions” occur.
 Jong-Hoon Nam, Assistant Professor of Mechanical Engineering and of Biomedical Engineering, hopes to provide answers to this and other mysteries of our incredibly complex sense of hearing with an NIH grant that could total $1.8 million over the next five years.
Jong-Hoon Nam, Assistant Professor of Mechanical Engineering and of Biomedical Engineering, hopes to provide answers to this and other mysteries of our incredibly complex sense of hearing with an NIH grant that could total $1.8 million over the next five years.
His lab will combine ambitious computer simulations with a novel microfluidic chamber to focus specifically on the organ of Corti. The organ -- a complex, truss-like strip consisting of inner and outer hair cells, a basilar membrane and supporting cells --plays a key role in converting sound-generated oscillations in the cochlea’s fluid-filled chambers into electrical signals that go to the brain. Indeed, damage to the outer hair cells is a “prominent signature” of most types of hearing loss, Nam said.
“The biomechanics of the organ of Corti have been under-investigated,” Nam noted. “We would like to know how the complicated structure of the organ of Corti contributes to the overall function of the cochlea. That’s the major theme of this proposal.”
He hopes his study will lay groundwork that eventually will lead to hearing aids or implants that are better customized to the needs of individual patients. There could be other applications as well. The inner ear can process a wide range of both frequencies and loudness; a better understanding of how it does this might lead to more sensitive pressure transducers and other engineering applications.
“Engineers continually obtain ideas from biological systems,” Nam noted. “And the inner ear is a beautiful sensor, operating over a remarkably wide range.”
Much of the research on the inner ear to date has occurred at two extremes of scale: the macro biophysics of the cochlea as a whole, and the physiology of individual cells and molecules. By focusing on the multi-cellular physics of the organ of Corti, and the electro-mechanical interaction between outer hair cells and the microstructures around them, Nam hopes to “bridge” these previous findings and provide a “new integrative paradigm of hearing research.”
Specifically, he would like to explore three hypotheses:
- Two different tuning mechanisms explain our ability to hear a wide range of frequencies, involving fluid inertia and tectorial membrane stiffness at the cochlea’s apex, and organ of Corti mass and basilar membrane stiffness at the base.
- Active hair cells modulate power dissipation within the inner ear for optimal amplification and tuning of sounds. Because of the high viscosity of the fluid in the inner ear, the energy of sound vibrations would dissipate too quickly to activate nerve signals to the brain. Much research has focused on how outer hair cells play a key role in amplifying this energy. Nam hopes to clarify how the dissipation of this energy – an “underappreciated but crucial aspect”— occurs to illustrate the overall balancing act that occurs within the cochlea.
- Otoacoustic emissions originate from an imbalance between the power generated by outer hair cells and dissipated by the entire organ of Corti complex.
“We have a long-term ambition to explain some hearing abnormalities as resulting from an imbalance between dissipation and compensation mechanisms in the organ of Corti,” Nam said. “This project will be an essential step toward that goal.”
To explore these hypotheses, Nam and his lab will employ novel experimental and computational approaches.
NSF funding supported Nam’s development of a microfluidic chamber that imitates the physiological conditions of the cochlea. This will help Nam’s lab use tissue from rodent models to “address the pivotal question in cochlear research—how outer hair cells, the cochlear amplifier, work within the organ of Corti.”
NSF funding also helped Nam develop a multi-scale computational model that incorporates the kinetics of the mechano-transduction channels, the mechanics of the hair-bundle, the electrical circuit of hair cells including their motility, the fluid mechanics of IHC, the electro-mechanics of the organ of Corti and the entire cochlea. The Nam group developed all the computer codes by themselves because no commercial program provides an easier way to solve such multi-scale and multi-physics problems.
Their preliminary computational work was supported by a Health Sciences Center for Computational Innovation (HSCCI) award through the University of Rochester Medical Center.
Nam says the NIH funding will support involvement of two to four PhD students in the project. Co-PI is Joseph C. Holt, Assistant Professor in the Department of Otolaryngology, who will provide advice on the pharmacological aspects of the organ of Corti’s physiology.
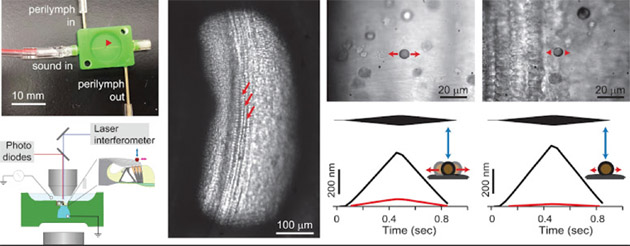
At far left is the microfluidic chamber developed by Asst. Prof. Jong-Hoon Nam that imitates the physiological conditions of the cochlea. This will help Nam’s lab use tissue from rodent models (shown at left center) to address the pivotal question in cochlear research—how outer hair cells, which serve as the cochlear amplifier, work within the organ of Corti.
