A Rochester Scholars photo gallery
Day Three: Reaction engineering
“Chemical reaction engineering is at the heart of virtually every chemical process,” Professor Tenhaeff explained in his lecture. When scaling up chemical reactions to industrial scale, chemical engineers combine their knowledge of chemical kinetics with thermodynamics, heat and mass transfer, and economics “in order to produce the required amount of product safely, efficiently, and as cheaply as possible.”
They use rate laws to help determine what kind of reactor will be used. The three common industrial reactors are: batch reactors (up to 100,00 tons per year), continuously stirred tank reactors (up to 3 million tons per year), or plug flow tubular reactors (up to 5 million tons per year).
Temperature control is vital; if an exothermic (heat generating) reaction is involved, sufficient safeguards must be built in to eliminate the possibility of a thermal run-away. Tenhaeff showed a U.S. Chemical Safety Board safety video depicting a tragic reactive chemical accident that devastated the T2 Laboratories in Jacksonville, Florida.
In the lab, students mixed crystal violet solution with sodium hydroxide in a batch reaction, then used computer-interfaced colorimeters to study reaction kinetics using the relationship between reactant concentration and optical density.

Throughout the week, students split into teams of three members each to conduct their experiments – just as they would work in teams as chemical engineers. From left to right: Samantha H., Taylor D., and Riley W.
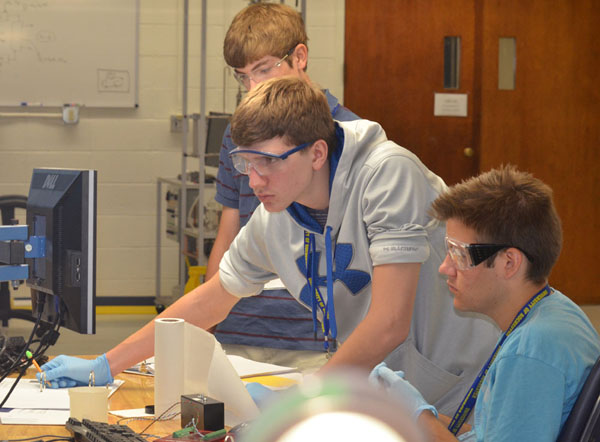
Alec B., William B. and Chad C. (above) and Garrett H., Lukas J. and Aaron G. (below) peer at their computer screens as their colorimeter, in conjunction with a LabView software program, records changing values as different concentrations of sodium hydroxide react with crystal violet solution.


Austin P. jots down optical density readings every 10 seconds during trials involving each of two concentrations of sodium hydroxide.
