Prof. Kelley's lab studies role of mixing in performance of liquid metal batteries
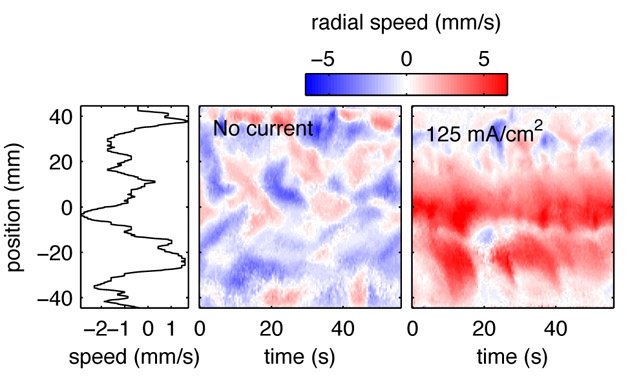 Fluid flow in a liquid metal electrode, measured with ultrasound. Reds indicate flow away from the probe, and blues indicate flow toward it. Adding electrical current causes a global rearrangement of the convection pattern and makes mixing more efficient.
Fluid flow in a liquid metal electrode, measured with ultrasound. Reds indicate flow away from the probe, and blues indicate flow toward it. Adding electrical current causes a global rearrangement of the convection pattern and makes mixing more efficient.
Traditionally, a published research paper has meant just that: a peer-reviewed written summary with a lot of text, footnotes and perhaps some illustrations.
But there will be an additional feature when a paper from Asst. Prof. Doug Kelley’s lab appears in the Journal of Visualized Experiments this summer.
The publication of “Ultrasound velocity measurement in a liquid metal electrode” will include a professionally rendered video to show how the measurements are done.
“They sent a film crew to the lab, which is pretty slick,” said Kelley, who appears in the video several times. Being interviewed by a film crew was no big deal, he added. “It was about like giving a talk.”
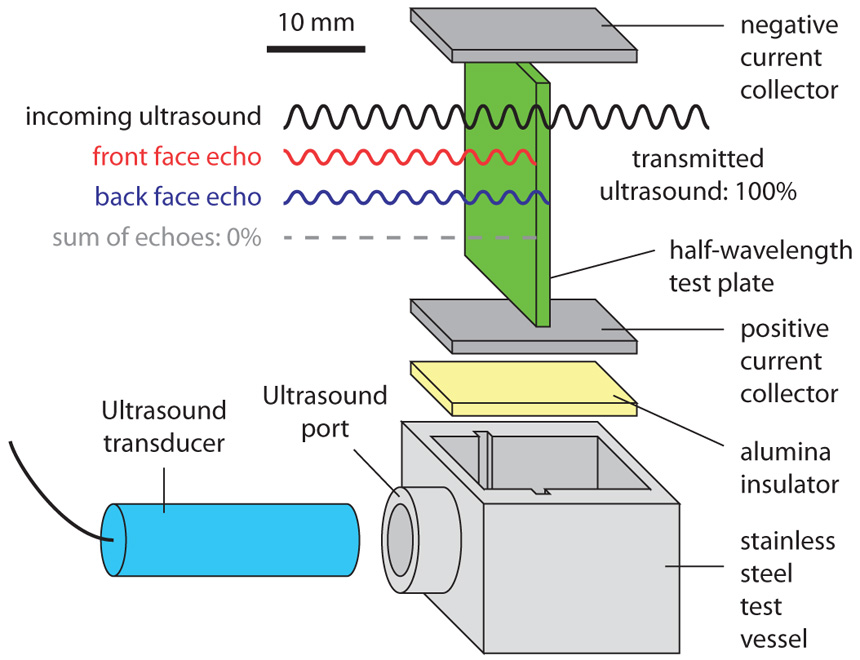
The “method” paper, by Kelley and first author Adalberto Perez ’15, describes an experimental apparatus that uses ultrasound to measure the flow patterns and velocities of the opaque fluids used in liquid metal electrodes.
This could help resolve one of the obstacles to creating liquid metal batteries that are large enough – and sufficiently cost-effective -- to store surplus electricity for grids that power entire communities or regions.
“Currently these grids have almost no storage,” Kelley explained. “The electricity you use was generated a fraction of a second before you use it. It comes straight to you, on demand. That makes grids complicated to manage, because somebody somewhere, 24 hours a day, has to be turning generators on and off to match our usage.” This is especially problematic with renewable wind and solar energy, which can be interrupted by calm or cloudy weather.
Liquid metal batteries offer one approach to inexpensive energy storage on a large scale, Kelley said.
Unlike traditional batteries, they do not have solid electrodes. They consist of two layers of liquid metal from different parts of the periodic table – for example lithium above, lead and antimony below -- separated by a layer of molten salt, which acts as the electrolyte.
Liquid metal batteries have the potential to last much longer than solid state batteries, which tend to swell and shrink with each charge or discharge. “Slowly but surely they grind themselves to dust over time. But when everything is liquid, that doesn’t happen,” Kelley noted.
The main problem with liquid metal batteries has been keeping the chemistry uniform in containers large enough to power entire grids.
That’s where Kelley’s lab comes into the picture. “I think we could solve this problem with better mixing,” Kelley said.
The first step is characterizing how that mixing is affected by various fluid flow patterns and velocities, which Kelley’s lab is simulating with the experimental apparatus shown below.
“We’re working toward building empirical models, where we could say with reasonable probability, if the battery is discharged, and you’re running this current, this is what the flow is likely to be – and then start putting in strategies to improve it.”