ChemE researcher helps develop next-generation batteries, large and small
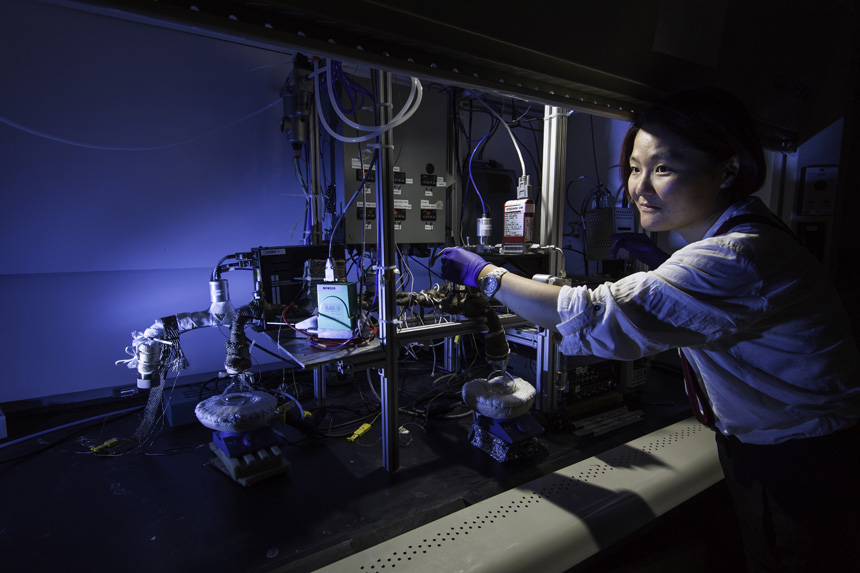
Yifan Gao, PhD student in the lab of Wyatt Tenhaeff, assistant professor of chemical engineering, works with a iCVD (initiated chemical vapor deposition) reactor, which will be used to synthesize solid electrolytes for 3-D microbatteries.
A University of Rochester researcher is helping develop next-generation batteries that will be small enough to fit into clothing – and others that will be large enough to power an electric vehicle.
If Wyatt Tenhaeff and his colleagues succeed, miniature batteries would expand the use of personal fitness trackers, implantable medical devices, active RFID tags to track pets and objects, and other small devices connected to the Internet.
And a lithium battery in a car would no longer carry the risk of catching on fire.
Tenhaeff, an assistant professor of chemical engineering, is applying his expertise in polymer electrolytes to three projects --playing different roles at very different scales of engineering.
As a principal investigator, he has received a $306,000 grant from the National Science Foundation to see if a chemical vapor deposition process he's developed can overcome the biggest challenge to 3-D microbatteries.
And he’s collaborating with researchers from five other institutions and companies on two ARPA-E grants. One, for $3.5 million, is to develop scalable manufacturing processes for ceramic electrolytes to be used in solid-state lithium metal batteries for electric vehicles. The other, for $3 million, will attempt to find glassy lithium-ion conductors that are stable and can be fully integrated into battery cells at a low cost.
In all, these projects will provide $956,000 of external funding to support research in Tenhaeff's lab.
Smaller sensors require smaller batteries
As sensors become smaller and smaller, the challenge is to proportionally shrink the batteries they require. “The real limitation to further miniaturization of MEMS (micro-electromechanical devices) and micro-sensors is the power source,” says Tenhaeff.
For years, researchers have been working to develop ever smaller, thin film lithium ion batteries. They are fabricated using processes akin to semiconductor processing, in which the anode (the positively charged electrode), cathode (the negatively charged electrode) and electrolyte (the material that allows electricity to flow from one electrode to another) are laminated as thin layers.
The challenge is generating sufficient energy densities at such a small scale. One approach is to put these thin film structures on 3-D platforms, which increases the surface area. “The challenge plaguing the 3D microbattery research field, however, has been the preparation of ultrathin solid electrolytes with sufficient conductivity,” Tenhaeff says. Ultrathin refers to layers that are less than 100nm in thickness.
The problem, he believes, is that most research has focused on synthesizing solid electrolytes from a liquid state. “This makes it very difficult to control the uniformity and thickness of the electrolyte coating– especially on complex, aperiodic electrode topographies,” Tenhaeff says.
Instead, with the National Science Foundation grant he was recently awarded, he will attempt to show that a process called initiated chemical vapor deposition (ICVD) can solve this problem. “ICVD gives us better control because we are growing a solid polymer electrolyte from the gas phase. We don’t have to worry about surface tension effects, and can generate highly conformal ultrathin coatings, meaning that the entire surface is coated uniformly.”
The grant will fund a PhD student and an undergraduate to work with Tenhaeff on developing a deposition process that can produce the optimal balance of film composition, mechanical properties, and ion conductivity, then use it to produce 3-D battery cells that are mere millimeters in size.
In addition to powering microsensors, miniature 3-D batteries could be useful in other ways. For example, when woven into the fabric of a soldier’s uniform they could provide a power source for high tech battlefield tools – at the same eliminating the need to burden the soldier with a more cumbersome battery.
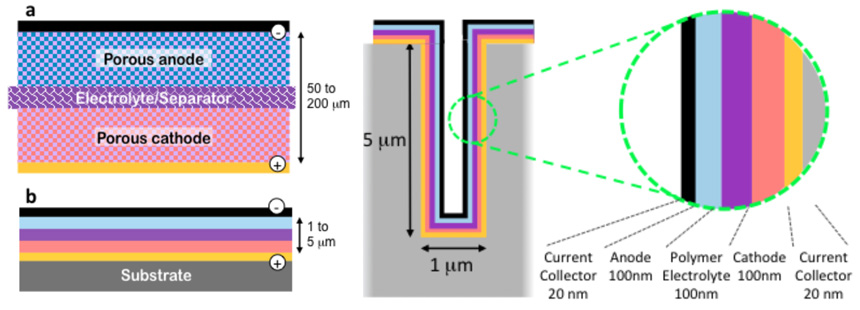
These illustrations from Tenhaeff’s lab compare a conventional lithium battery with electrolyte (figure a at upper left) and a lithium ion thin film battery (figure b, lower left). At right, a cross section of a 3-D microbattery.
A safer lithium battery for vehicles
Tenhaeff is also collaborating on a $3.5 million project to develop solid-state lithium metal batteries for vehicles that feature a solid ceramic electrolyte in place of the combustible liquid electrolytes now used.
The project, funded by ARPA-E, the U.S. Department of Energy agency that promotes advanced energy technologies, is led by Jeff Sakamoto, an associate professor at the University of Michigan. Tenhaeff previously collaborated with Sakamoto while working as a staff scientist at Oak Ridge National Laboratory prior to joining the University of Rochester.
Tenhaeff’s mentor at Oak Ridge, Nancy Dudney, a leading expert in solid state lithium battery design, is also collaborating on this project, along with researchers at the Ford Motor Company and the Army Research Laboratory.
The team is using a highly conductive form of a lithium-bearing garnet for the electrolyte. And therein lies part of the challenge, Tenhaeff says. High temperatures – 1,100 to 1,200°C– are required to produce the garnet with the correct crystalline structure. So the goal of the project is to develop processes for fabricating the material, then integrating it with other battery components in a way that will make large scale manufacturing feasible.
Tenhaeff is receiving a $275,000 share of the funding to develop polymer electrolytes to be integrated into the design. “Polymers are easier to process at low temperatures,” Tenhaeff said. “By integrating polymer electrolytes and ceramic electrolytes, we can come up with a structure that we can manufacture readily.”
Tenhaeff will use part of the funding to support a PhD student to work on the project.
A quest for glassy lithium-ion conductors
Tenhaeff is also collaborating with Oak Ridge researchers on a $3 million ARPA-E project to find glassy lithium-ion conductors that are stable and can be fully integrated into battery cells at a low cost.
Glassy electrolytes were chosen for their ability to withstand many charging cycles while preventing the formation of lithium dendrites at higher currents. Glass also provides a flexible platform for experimenting with a variety of different lithium-rich materials. Advanced glass processing will allow for a range of different structures and materials while remaining cost effective to produce.
Tenhaeff, whose $375,000 share will help fund a post-doc and possibly a masters student as well, will work on developing materials that will allow integrating the glassy lithium-ion conductors with conventional composite (porous) cathode materials and provide mechanical reinforcement.
This grant is one of 16 awarded nationwide as part of ARPA-E's IONICS program. By creating high performance parts built with solid ion conductors – solids in which ions can be mobile and store energy – the IONICS program seeks new ways to process and integrate these parts into devices with the goal of accelerating their commercial deployment. In particular, IONICS projects will work to improve energy storage and conversion technologies in three categories: transportation batteries, grid-level storage, and fuel cells.
Wyatt Tenhaeff, assistant professor of chemical engineering, with lab members (from left to right) Zhuo Li, Christina Engler, Marina Ioanniti, and Yifan Gao.
