Meet The Team!

Supervisor

Stephen McAleavey, Ph.D.
Associate Professor of Biomedical Engineering
Associate Professor of Electrical and Computer Engineering
Rochester Center for Biomedical Ultrasound
Chair, Department of Biomedical Engineering
Customers

Alayna E. Loiselle, Ph.D.
Associate Professor of Department of Orthopaedics, Center for Musculoskeletal Research (SMD)
Associate Professor of Department of Biomedical Engineering (SMD) – Joint
Associate Professor of Department of Pathology and Laboratory Medicine (SMD) – Joint

Constantinos Ketonis, M.D., Ph.D.
Orthopedic Hand Surgeon
Background
Flexor tendons enable fine motor control by transmitting muscle force from the forearm muscles to the fingers, allowing precise and coordinated finger movement. Injuries to these tendons often require surgical repair but are frequently complicated by scar tissue (adhesion) formation, which restricts range of motion, even when the repair is successful.
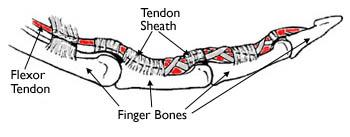
Problem Statement
Present methods for managing flexor tendon injuries lack the ability to monitor adhesion formation effectively, resulting in limited range of motion and suboptimal patient treatment outcomes.
Working in collaboration with our customers, Dr. Loiselle and Dr. Ketonis, this design aims to automate the capture of ultrasound images and reconstruct them into a 3D model of the tendon, enabling precise visualization of scar tissue to improve healing and recovery.
Customer Scenario
Our device will mainly be used in clinics by health care professionals for patients with scar tendon injury pre/post surgery as a tool to better understand scar adhesion formation, and improve patient outcomes.
Design
Requirements
- The system must be automated to ensure consistent and reliable images of tendon and scar tissue across different users and patients
- A custom water bath is needed to provide a consistent coupling medium for ultrasound imaging, eliminating the need for continuous gel application
- The system must be capable of capturing axial images that can be stacked to create a 3D model, allowing for visualization of tendon and scar tissue. This will help to gain accurate diagnosis and future treatment planning
- The system must adapt to different patients, considering that many patients have limited range of motion due to scar formation or other conditions. Additionally, the system must have the ability to accommodate y-directional changes based on the constraints of the patient.
- Integrated y-direction current threshold is necessary to prevent excess pressure on the patient’s hand and should automatically retract once a certain current threshold is passed.
Mechanical Specifications
- The overall system dimensions are 18in x 17in x 21.2in (length x width x height)
- The linear actuator can extend up to 4 inches
- The water bath dimensions are 13.5in x 9in x 6in
Electronic Specifications
- A servo motor (MG996R) with 13 kg/cm torque is attached to the end of the actuator with arduino boards* and is used to “sweep” the probe attachment for various angles to accommodate patients with varying degrees of contractures
- A stepper motor is nestled above the linear actuator connected to arduino boards* for the x-direction.
- The linear actuator is also controlled by arduino boards* for the z-direction
* The boards used are the Arduino Uno R4 Minima, and the Arduino Motor Shield Rev3. The Motor Shield will control the motor that operates the movement in the x and z directions. The other board includes a microcontroller that allows the operator to talk to the system.
Software Specifications
- MATLAB is the software that is used to do 3D reconstruction and powers the GUI (app designer)
- The software connected to the butterfly probe to obtain images is an app designed by the Health Lab
Systems Overview
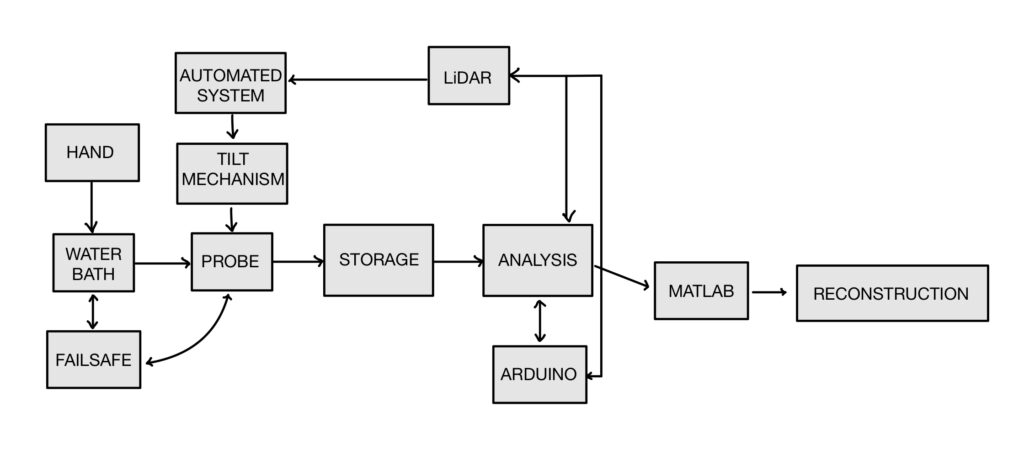
Our systems level design includes an external component, the patient’s hand, and internal components to see how they interact together.
Design of Prototype
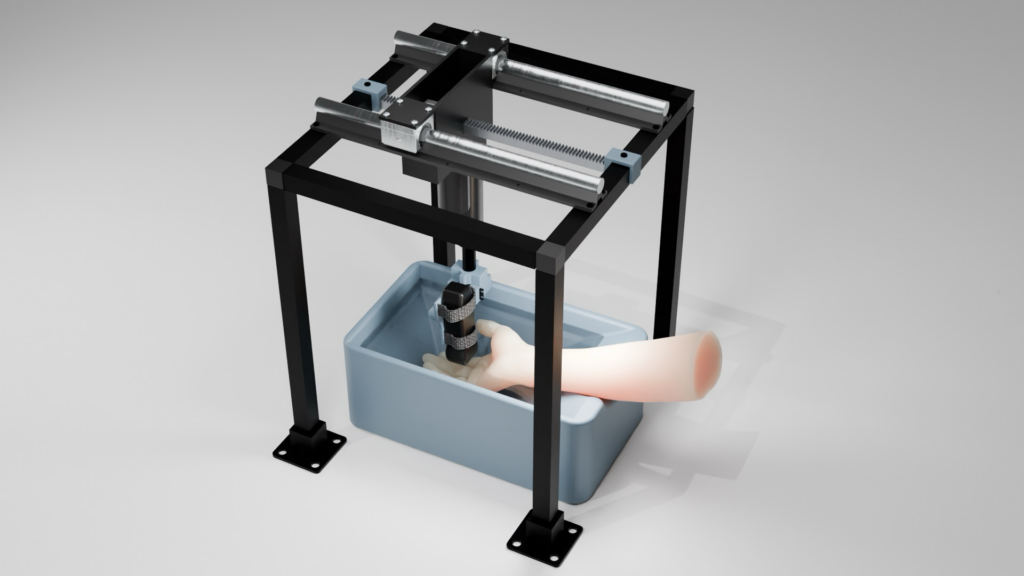
Rendered overview of design to scale using Blender.
Real Life Prototype
Software GUI + Reconstruction

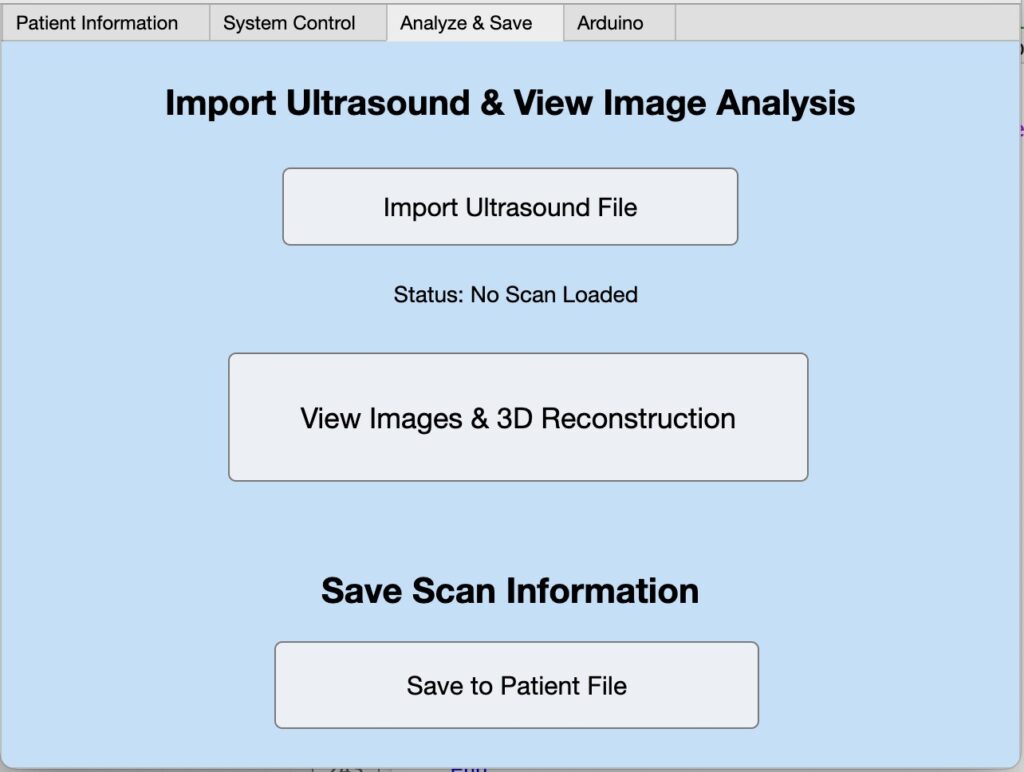
Future Direction
Moving forward, we want to implement a smoother user interface, more efficient scan speed, and be able to quantify the amount of scar tissue using AI.
Acknowledgements
We would like to thank Dr. Loiselle, Dr. Ketonis, Stephen McAleavey, Mark Buckley, Benjamín Castañeda, Ahmet Gurcan, Martin Gira, and Scott Seidman for their invaluable guidance, technical expertise, and ongoing support throughout the development of this project. Their insights played a crucial role in the design, validation, and refinement of our system. Also, a huge shoutout to our friends for their support throughout this process.

