Team
Team Sea Slug: Danielle Getz, Jakob Singer, Linnea Wegge, and Quinn Yu
Mentors
Melodie Lawton and Mark Juba (Department of Chemical Engineering)
Sponsors
David Cross and Nicole Simonetti (Waste Management)
Brian McGrath (Barton and Loguidice)
Project Background
What are PFAS, and why do we care about them?
Poly- and perfluorinated substances (PFAS) are man-made chemicals that have been widely used for industrial and residential applications over the last sixty years [1]. They are used in products such as non-stick cookware, surfactants, textiles, carpets, papers, paint, and fire-fighting foams [1]. After disposal of these products in landfills, PFAS contaminate landfill leachate and spread to surface water, groundwater, and soil [1]. PFAS have the potential to cause negative environmental consequences, including bioaccumulation in fish and other wildlife [2]. They also negatively impact human health by leading to increased cholesterol levels, high blood pressure, and increased risk of kidney cancer [3].
What is the motivation behind our project?
The Environmental Protection Agency proposed a new National Primary Drinking Water Regulation as of March 14, 2023 which will reduce the Maximum Contaminant Level of two PFAS compounds, PFOA and PFOS, to 4.0 ng/L [4]. These new limits will likely be enforceable by the end of 2023, and will likely lower the sewer use discharge limits of PFOA and PFOS, affecting permissible levels of PFAS in landfill leachate [4]. This has made it necessary for Team Sea Slug to investigate adsorption as a method to separate PFAS from landfill leachate for Waste Management.
Why did we choose adsorption using biochar?
PFAS do not break down in the environment due to their strong C-F bonds [1]. Adsorption is currently one of the leading implemented solutions for PFAS removal from aqueous solutions because of its low cost, technical feasibility, scientific consensus of high PFAS removal, and relative simplicity [5]. Biochar, made from the pyrolysis of biomass, is one potential material that can be used as an adsorbent. Waste Management receives about 8,000 tons of woody yard waste and 35,000 tons of wastewater treatment plant sludge per year, so Team Sea Slug postulated that one of these types of biomass could be pyrolyzed on-site to use as an adsorbent for PFAS in landfill leachate.
Methods
Which types of biochar were tested?
Sewage sludge biochar, purchased from a company in California, was tested with the logic that Waste Management could make biochar of a similar quality using their own wastewater sludge. Homemade biochar, made by Team Sea Slug using woody yard waste taken from the landfill, was tested as well as a softwood biochar sample found in the undergraduate chemical engineering lab. The softwood biochar was tested to compare its properties to the homemade biochar.
Surface Characterization
Team Sea Slug used a Scanning Electron Microscope (SEM) to understand the surface morphology of the different kinds of biochar, estimate pore size, and estimate the number of pores. The softwood biochar and homemade biochar had large pores, which suggested that they would be good adsorbents. The sewage sludge biochar had a more granular surface structure, meaning that it was likely a worse adsorbent.



Brunauer–Emmett–Teller (BET) analysis was used to estimate the total volume of pores, the surface area of the biochar, and the adsorption type. It was determined that softwood biochar is characteristic of a Type 2 isotherm, indicating that it is a good PFAS adsorbent.
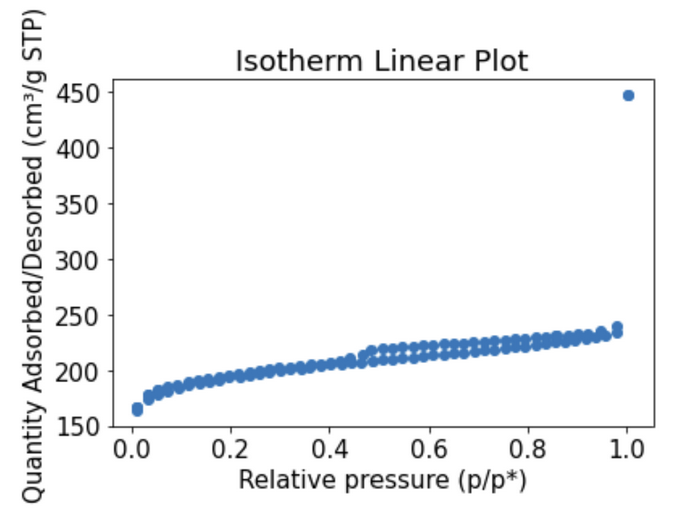
Adsorption Kinetics, Equilibrium Capacity, and Adsorption Isotherms
In order to further assess the quality of the biochar adsorbents, a series of batch experiments were performed in the lab to determine adsorption kinetics, equilibrium capacity, and create adsorption isotherms using PFAS concentration approximations. Surface tension of the was measured in the on campus lab and converted to PFAS concentration using a correlation created by Team Lululemon (2022) and further developed by Team Flamingo Tongue Snails (2023). These results suggested that softwood biochar was a good adsorbent based on the rate constant determined, amount of PFAS estimated to be adsorbed at equilibrium, and the Freundlich isotherm shape.
Making Biochar
Team Sea Slug created their own biochar in the lab on campus, using woody yard waste collected from the Waste Management landfill. This homemade biochar was created through pyrolysis, which is the thermal decomposition of material at high temperatures [6].


Results
Outsourced Lab Results
This graph shows the PFAS percent removal rate of two types of biochar- sewage sludge and homemade. Pure leachate was treated in a batch reactor containing biochar over a twenty-four hour period before being sent to Alpha Labs, an external PFAS concentration testing facility. Percent removals were calculated by comparing the post-treatment leachate samples to a control leachate sample taken at the beginning of the semester.
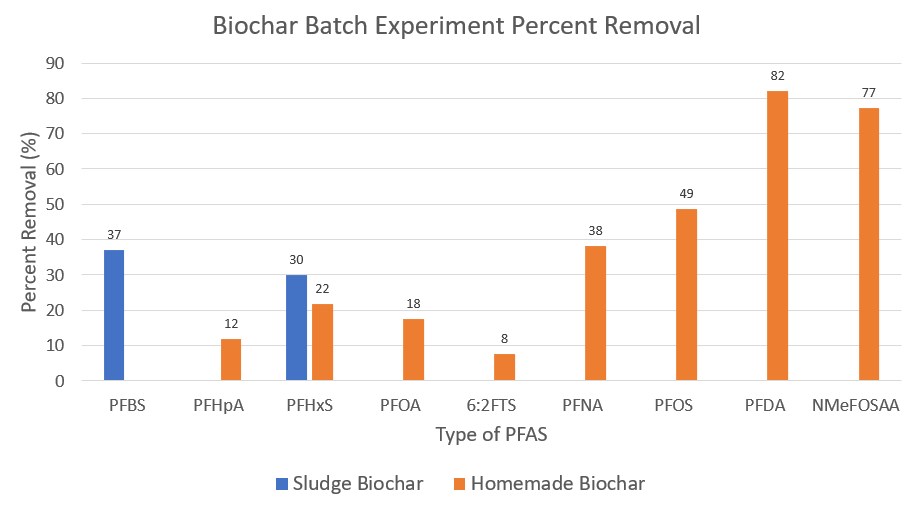
Eight types of PFAS were adsorbed by the homemade biochar. This included both PFOA and PFOS, two of the types targeted in the new proposed EPA regulations. This means that homemade biochar has the potential to treat leachate and help Waste Management meet the EPA regulations.
Sewage sludge biochar was only able to adsorb two types of PFAS, PFHxS and PFBS. However, both of these types are short-chain PFAS, which are more difficult to adsorb than long-chain PFAS.
Adsorption Scale-Up & Economics
Team Sea Slug suggests that Waste Management implement a counter-current mixer-settler system for adsorption using biochar at landfill scale. This system is similar to systems already implemented at wastewater treatment facilities.
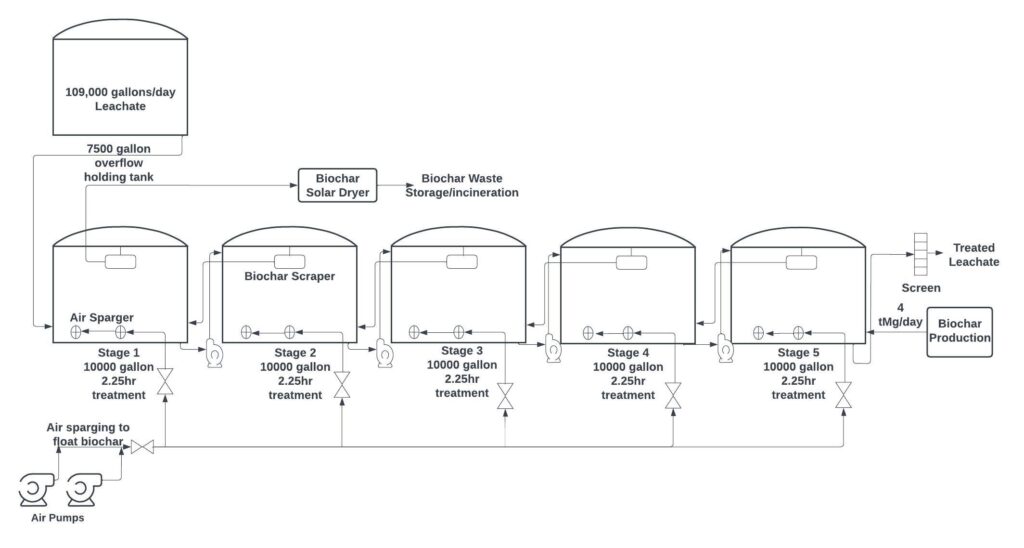
Based on an economic analysis done by Team Sea Slug, Waste Management can use this system to treat leachate for 2 cents per gallon if the biochar is homemade, or for 3 cents per gallon if the biochar is purchased from a local biochar producer.
Future Work
There are many possibilities for a future team to continue the work done by Team Sea Slug, including but not limited to:
- Developing a lab-scale version of the multi-staged counter current mixer-settler to compare lab-scale values with predictions
- Optimizing the pyrolysis process to improve the kinetics and equilibrium capacity of homemade biochar
- Developing methods of destruction or storage for the PFAS-saturated biochar
- Investigating hydrophilic biochar as a short-chain PFAS adsorbent
- Combining adsorption using biochar with the leachate foaming system that Team Flamingo Tongue Snails developed to remove more short-chain PFAS
Acknowledgments
Team Sea Slug would like to thank Brian McGrath from Barton & Loguidice, David Cross and Nicole Simonetti from Waste Management, and Prof. Mark Juba, Prof. Melodie Lawton, Prof. Doug Kelley, Clair Cunningham, Mason Garlatti, Jeffery Lefler, and TA Eliya Tashbib from the UR Department of Chemical Engineering.
References
[1] Singh, R. K., Brown, E., Thagard, S. M., & Holsen, T. M. (2021). Treatment of PFAS-containing landfill leachate using an enhanced contact plasma reactor. Journal of Hazardous Materials, 408, 124452.
[2] Centers for Disease Control and Prevention. (2022, May 2). Per- and polyfluorinated substances (PFAS) factsheet. Centers for Disease Control and Prevention. Retrieved March 28, 2023, from https://www.cdc.gov/biomonitoring/PFAS_FactSheet.html
[3] Centers for Disease Control and Prevention. (2022, November 1). Potential health effects of Pfas Chemicals. Centers for Disease Control and Prevention. Retrieved February 5, 2023, from https://www.atsdr.cdc.gov/pfas/health-effects/index.html
[4] Environmental Protection Agency. (n.d.). Proposed PFAS National Primary Drinking Water Regulation. EPA. Retrieved March 28, 2023, from https://www.epa.gov/sdwa/and-polyfluoroalkyl-substances-pfas
[5] Wu, C., et al. (2020). “Exploring the factors that influence the adsorption of anionic PFAS on conventional and emerging adsorbents in aquatic matrices.” Water Research 182: 115950. https://www.sciencedirect.com/science/article/pii/S2214714420302713
[6] “Oxford Languages and Google – English.” Oxford Languages, https://languages.oup.com/google-dictionary-en/.
