Meet the Team



BioDesign Statement
A device for anesthesiologists to detect unwanted infiltration and extravasation from delivery of intravenous fluids to reduce incidence of effects such as pain, swelling and risk of necrosis during the intraoperative period.
Summary
“Improve response times for infiltration and enhance workflow efficiency using DetectIV for continuous monitoring of IVs in real time”
DetectIV is a wearable, continuous monitoring medical device that aids anesthesiologists in the detection of infiltration/extravasation from delivery of intravenous fluids prompting early intervention. >200,000 hospital operating rooms and 35 million annual in-patient stays in the United States use IV therapy. Infrequent visual evaluation remains the current gold standard for monitoring IV sites. 50% of peripheral IVs failing results in adverse effects such as pain, swelling and tissue necrosis when left unattended.
Background
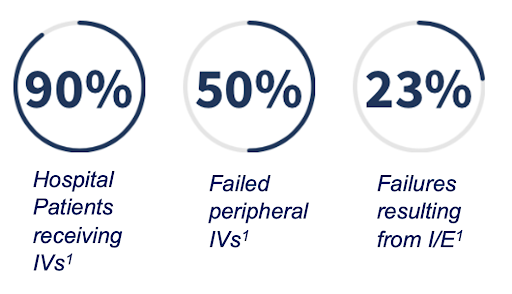
- >200,000 hospital operating rooms and 35 million annual in-patient stays in the United States use IV therapy.
- Infrequent visual evaluation remains the current gold standard for monitoring IV sites.
- Infiltration and extravasation occur when non-vesicant and vesicant fluids, respectively, leak out of the vein into surrounding soft tissue.
- Affected tissue may become swollen, cold to the touch, or discolored, but this may be not detected by the anesthesiologist if they do not have visibility of the site.
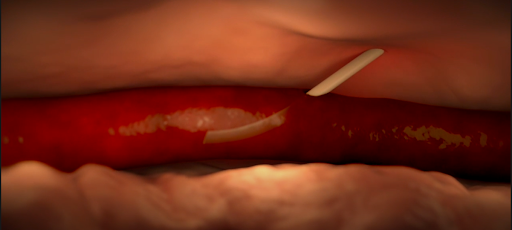
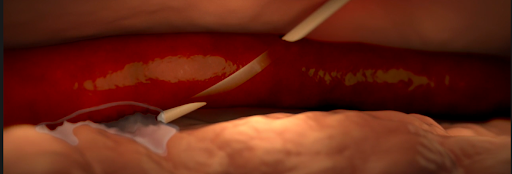
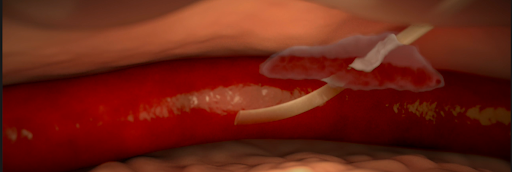
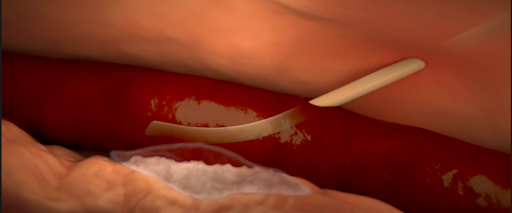
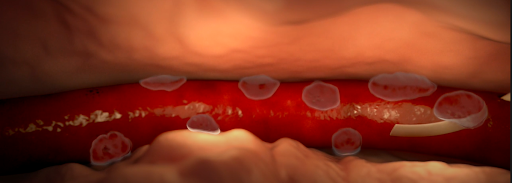
Various causes of infiltration and extravasation. [2]
Problem
- Visual evaluation of the IV insertion site is an ineffective method of infiltration diagnosis due to site inaccessibility during procedures.
- Undiagnosed infiltration can lead to adverse effects including temporary site pain, blistering, swelling, discoloration, compartment syndrome, and amputation of limbs.
- Malfunction of IVs prevent medication from affecting the body as intended, reducing the ability of the anesthesiologist to anesthetize, paralyze, and treat the patient effectively throughout the procedure.
Our Solution
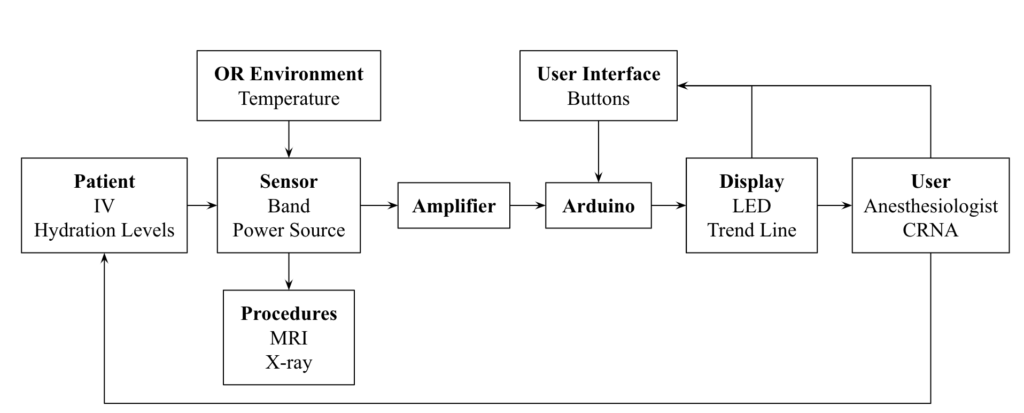
DetectIV intends to detect the presence of I/E by sensing a change in forearm size due to swelling. This will be implemented using an Arduino microcontroller and circuitry (Wheatstone Bridge and instrumentation amplifier): the resistances and voltages of the sensor increase as stretch/swelling increase; these changes will be used to determine if I/E has occurred. If detected, the monitor will emit a visual alarm to prompt the clinicians to inspect the IV site and proceed accordingly. The device model consists of a monitor with push button controls and LED indicators, a band with sensors and connection cables.
This provides an affordable, effective, and reusable way to ensure peace of mind that the IV is functioning properly even when the anesthesiologist does not have access to the IV site, minimizing hazards and costs to the hospital and patients.

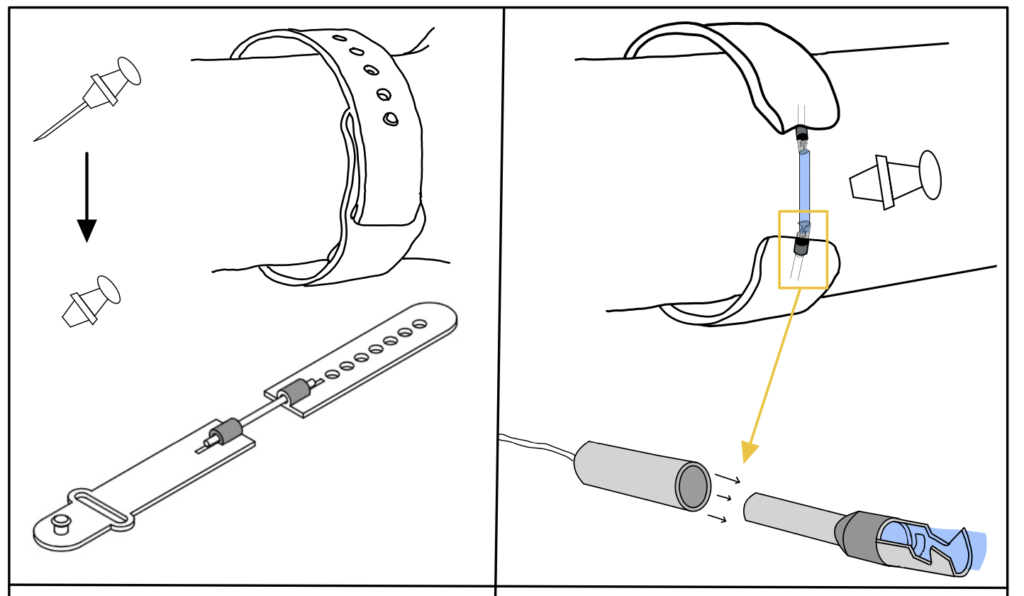
Sensor band is fastened to patient and the cables are attached to the sensor crimps. 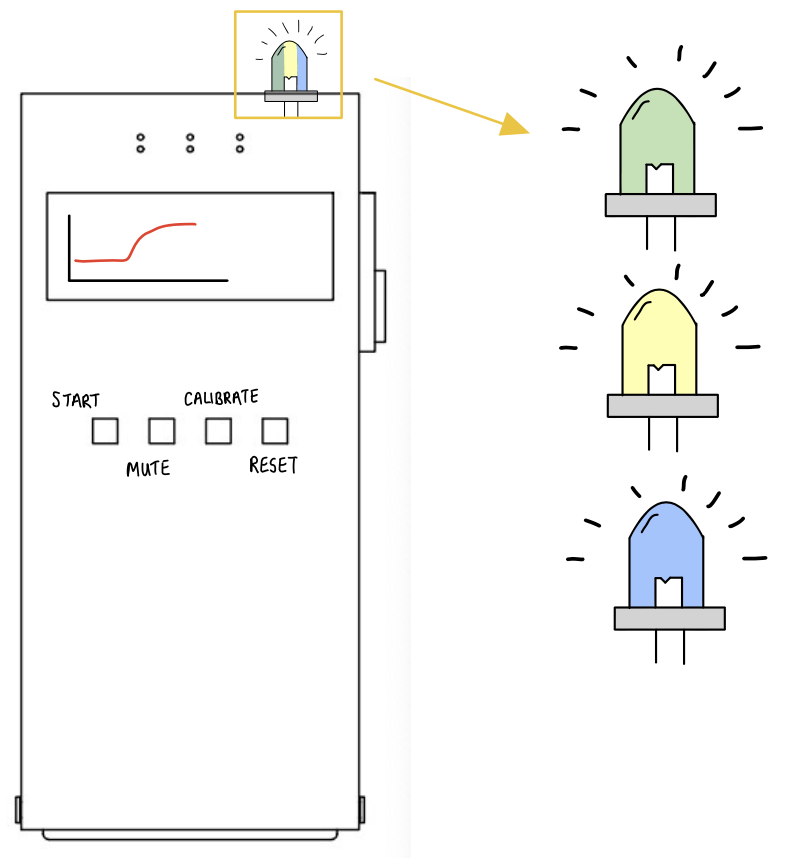
The top LED will change between yellow-green-blue to indicate the tightness of the band. 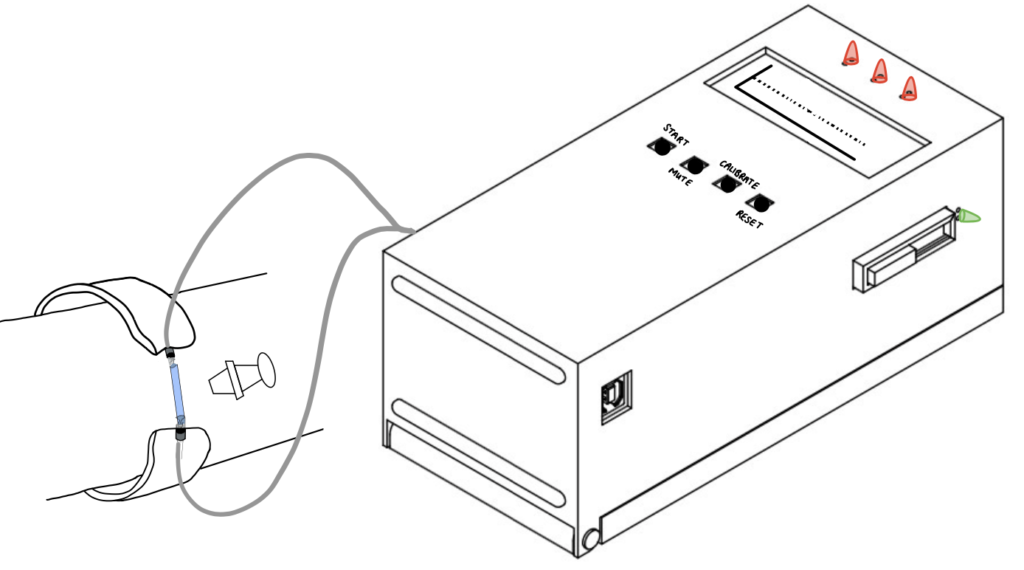
The monitor will display the sensor’s readings.
Subsystem Diagram
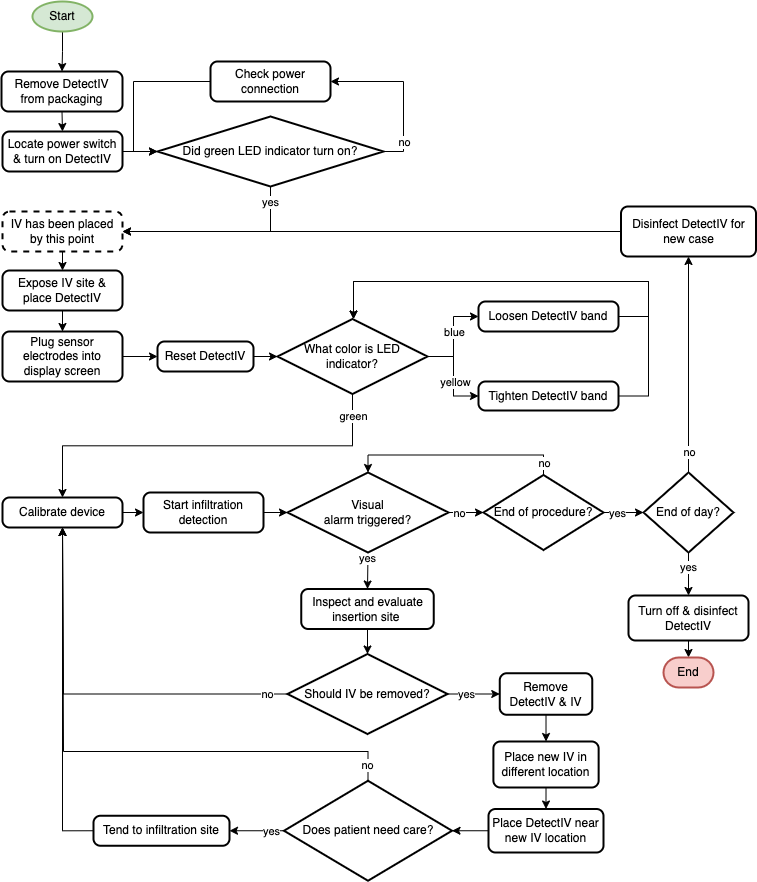
User Interaction Flowchart
Voice of the Customer Feedback

Benefits
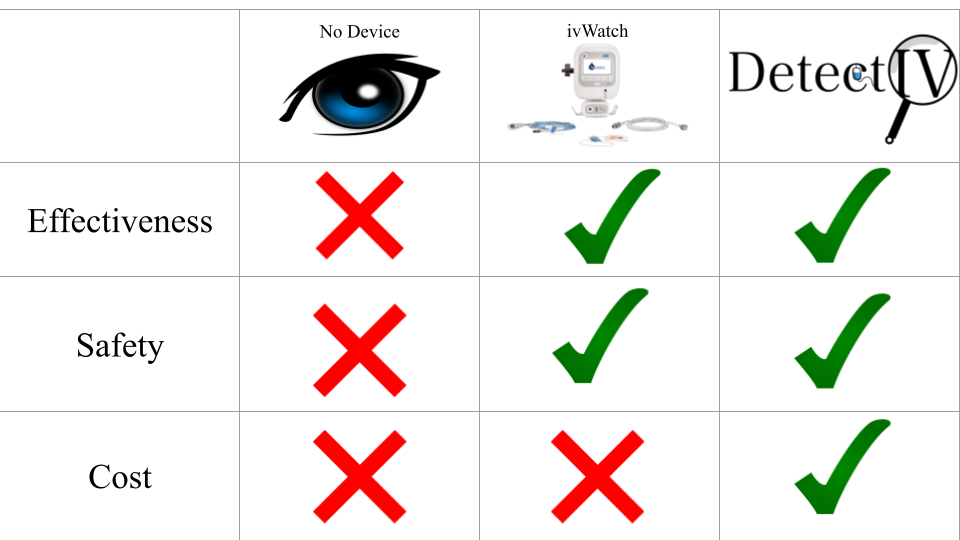
Competition
Testing
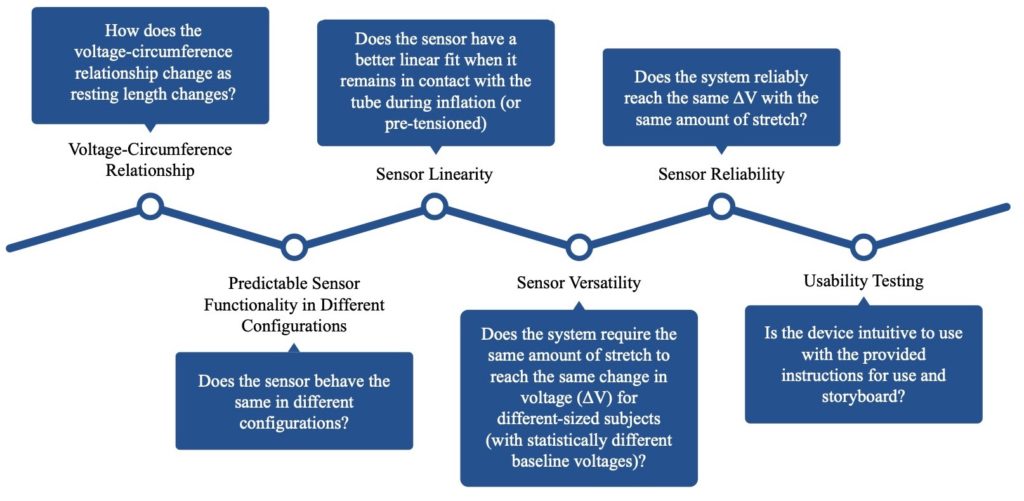
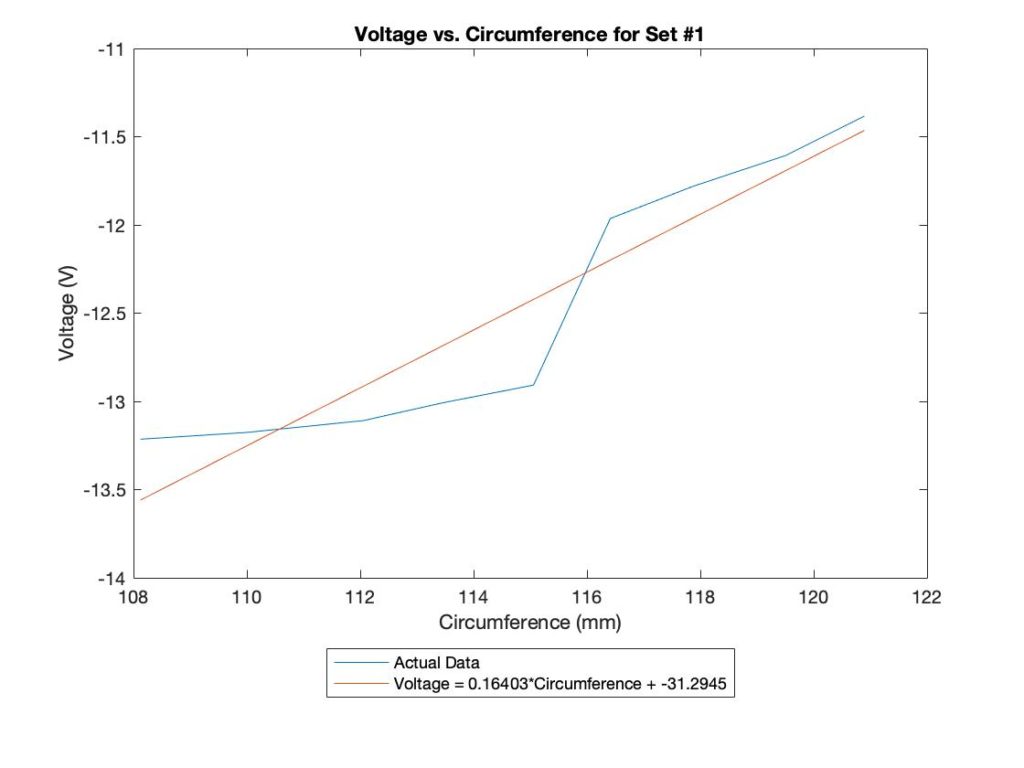
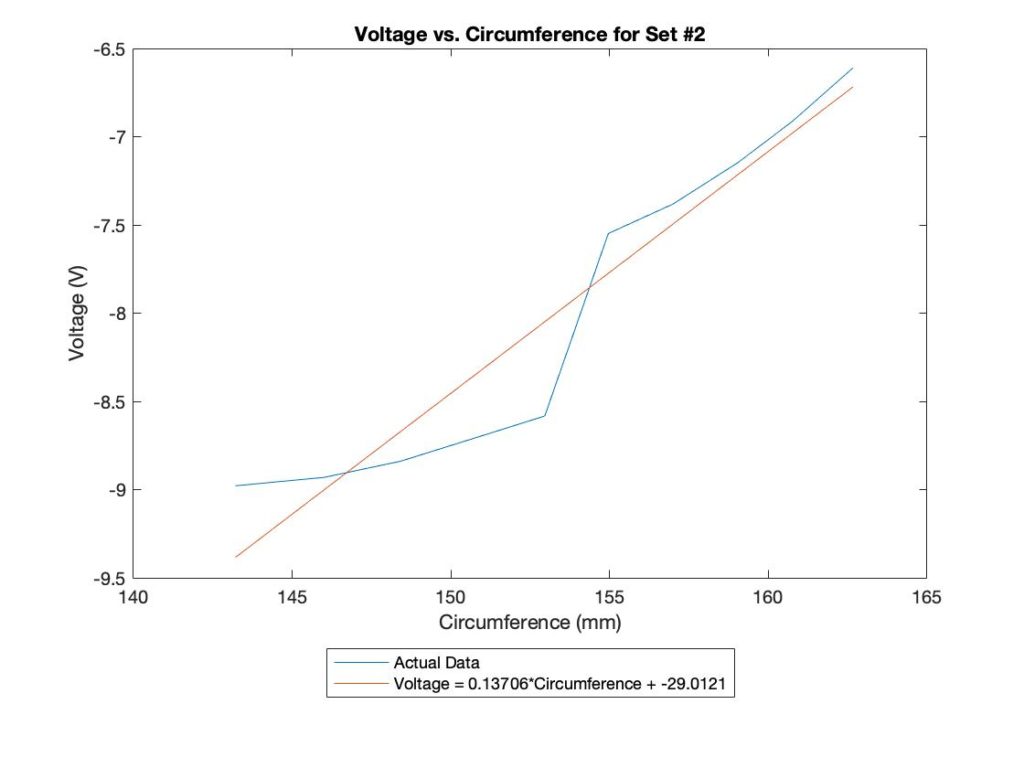
Voltage-Circumference Relationship
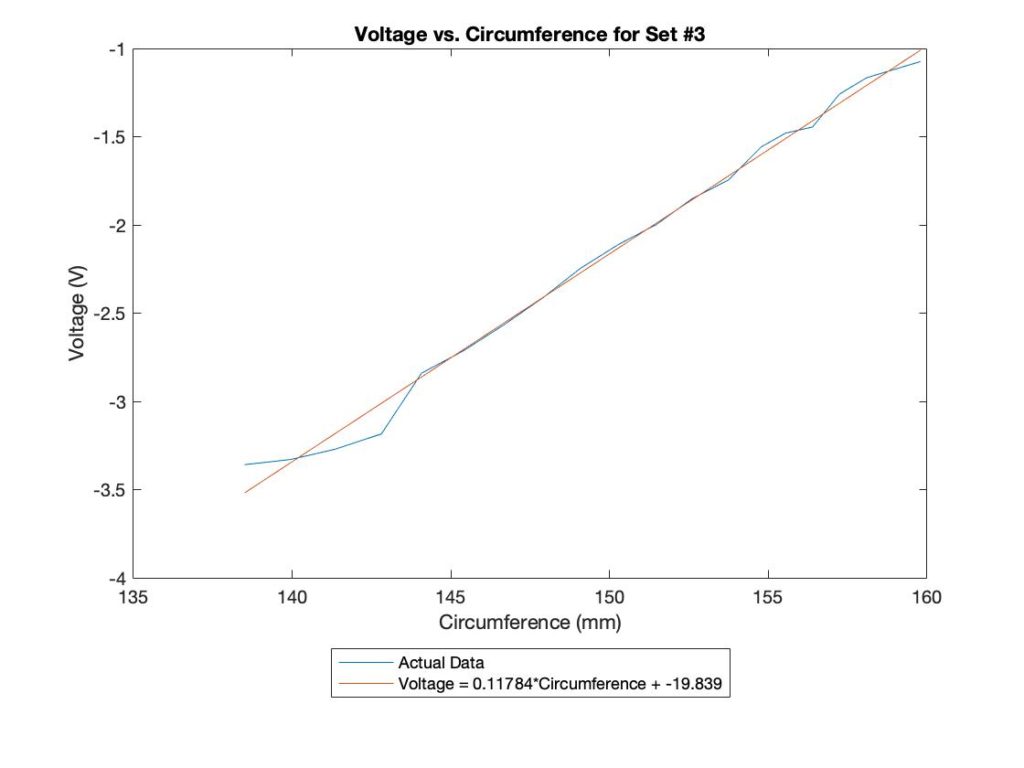
Predictable Sensor Functionality in Different Configurations
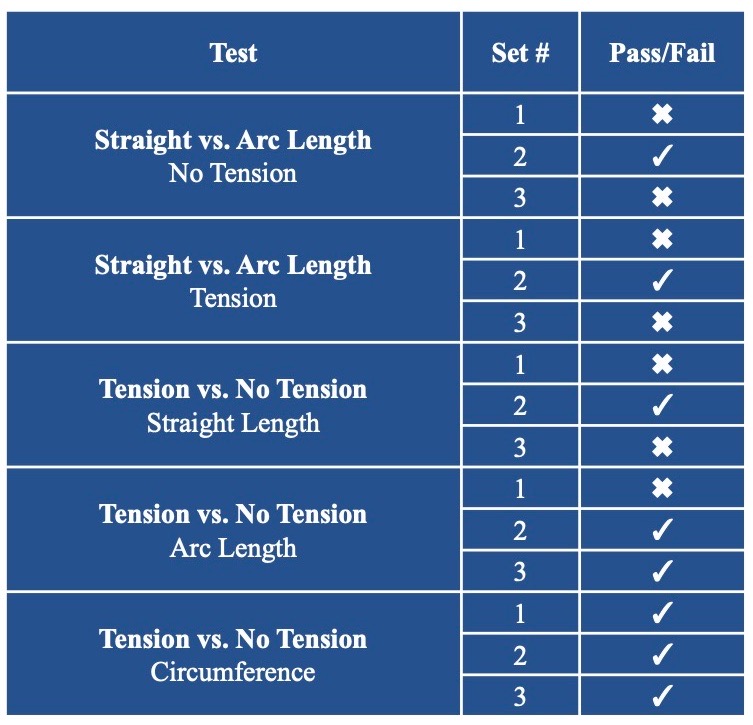
Sensor Linearity
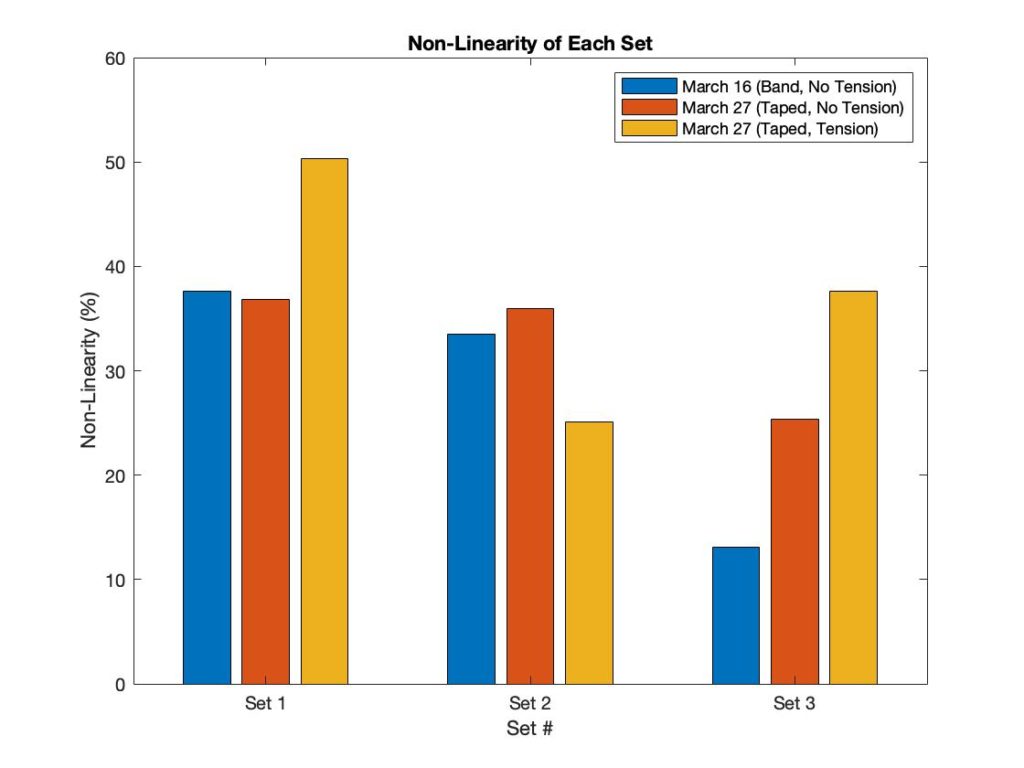
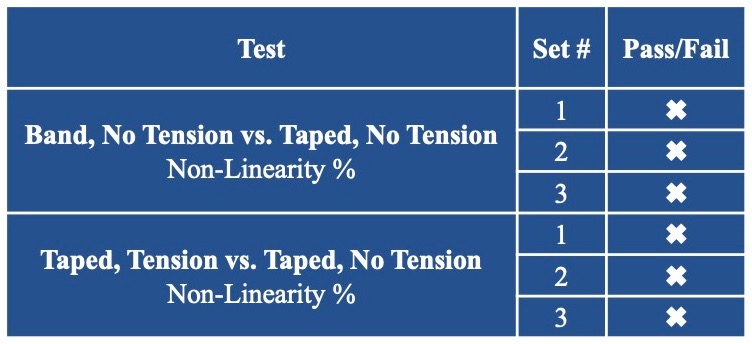
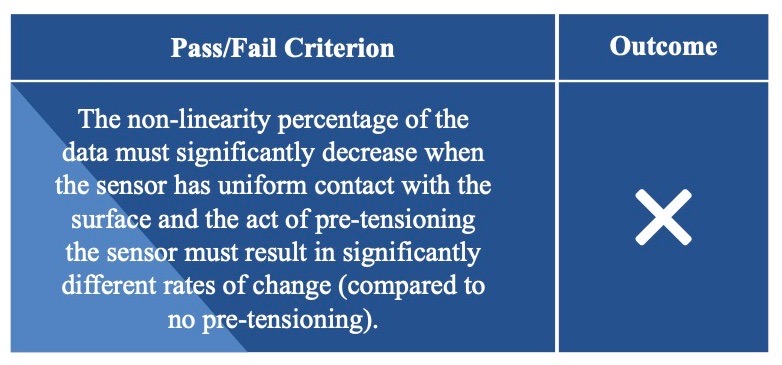
Sensor Versatility
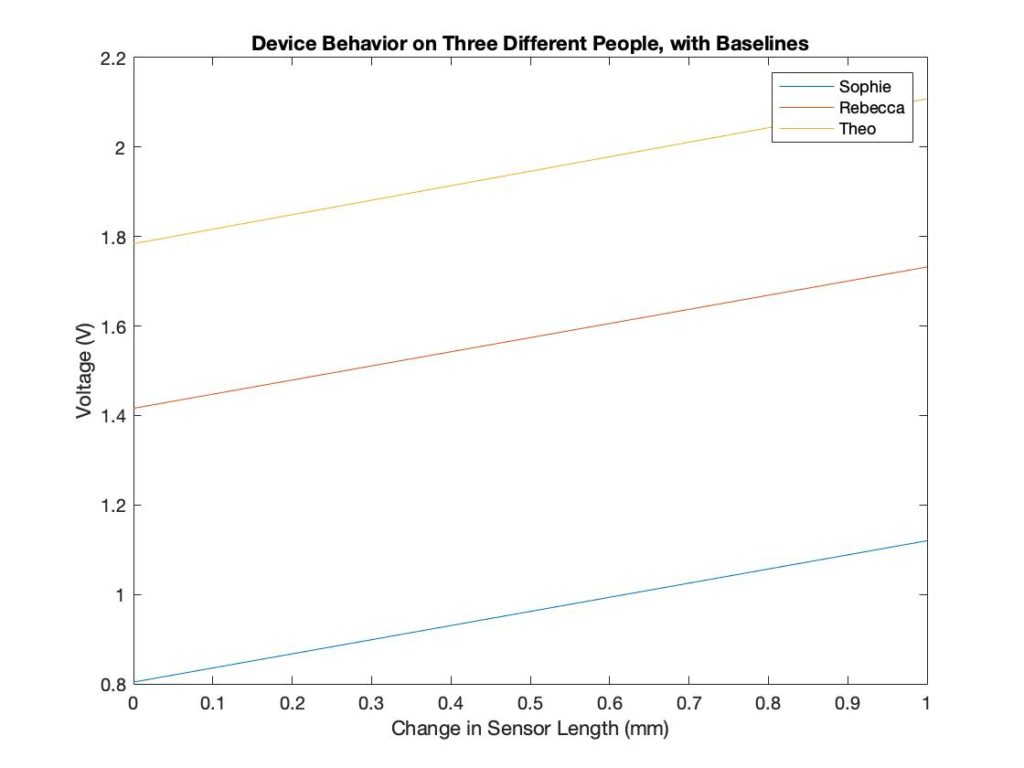
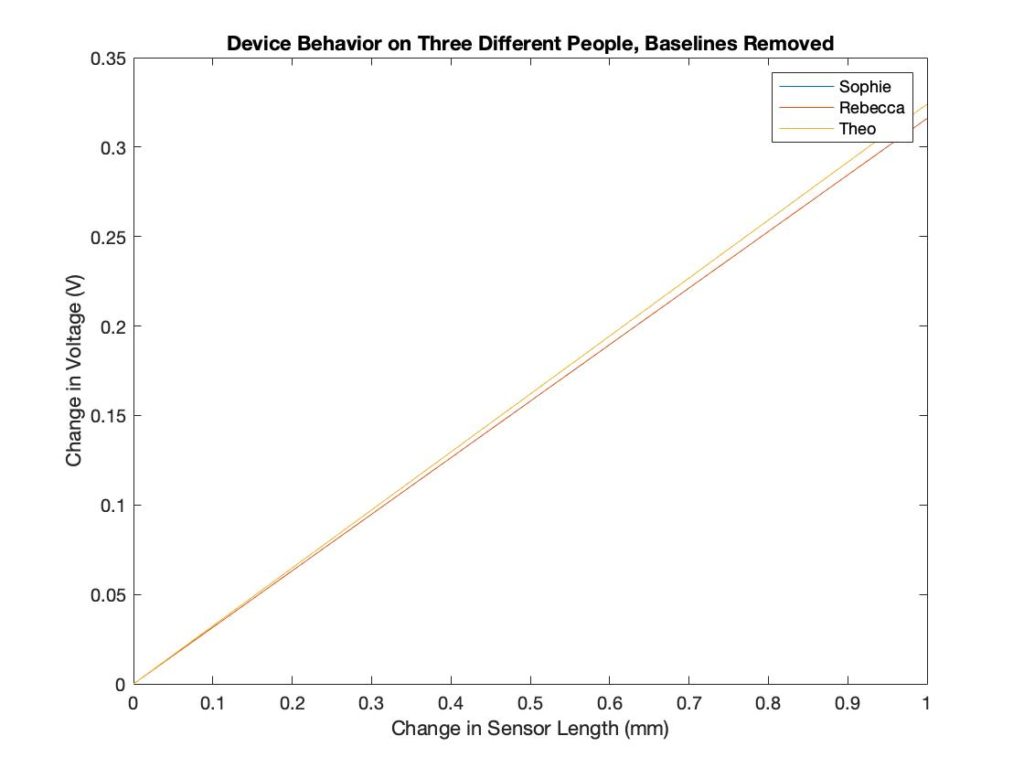
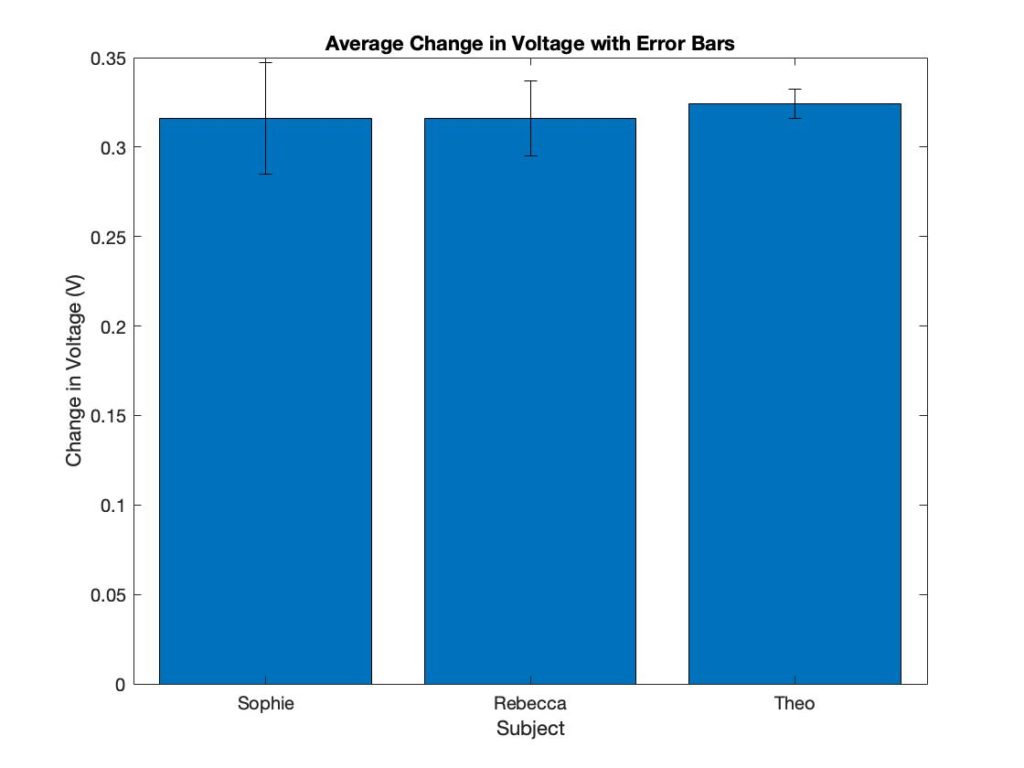
Each subject has a different forearm size, so the baseline measurements are different. The device calibrates according to the baseline and displays the difference from the baseline. The sensor outputs the same result, even when the baseline measurements were different. This shows that the device can be used on differently sized patients.


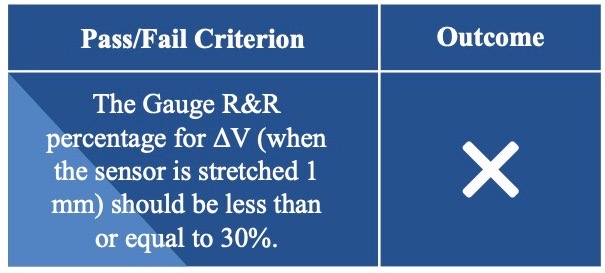
Sensor Reliability
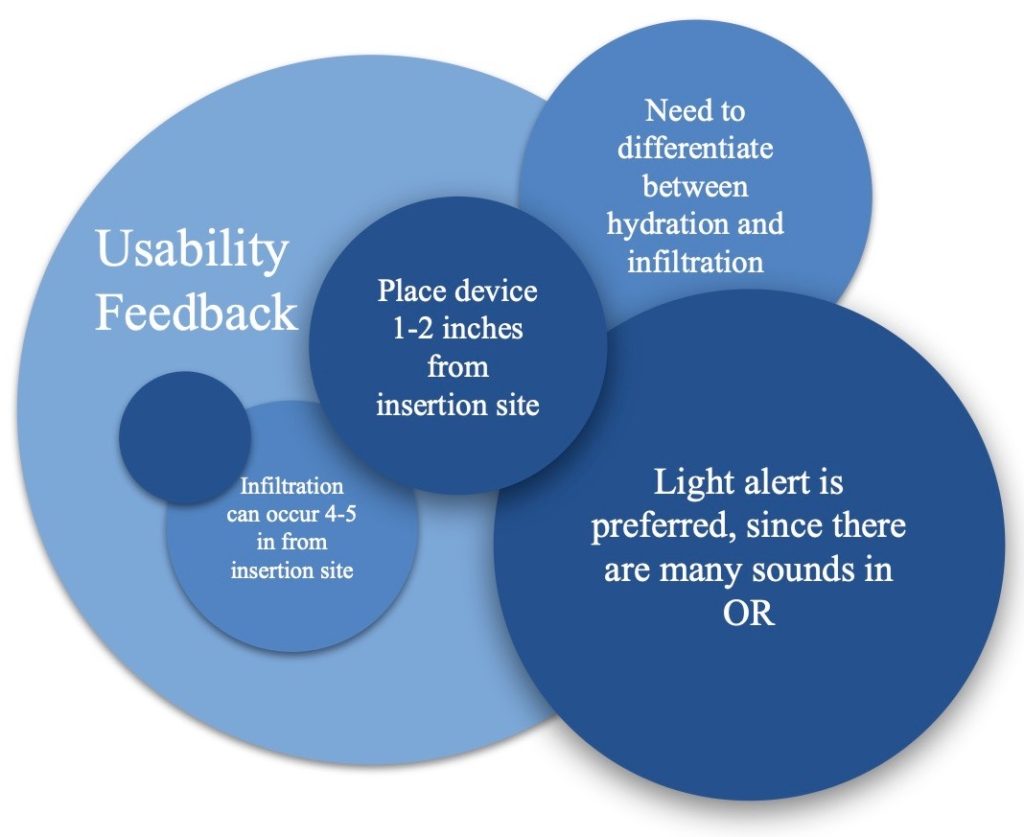
Usability Testing
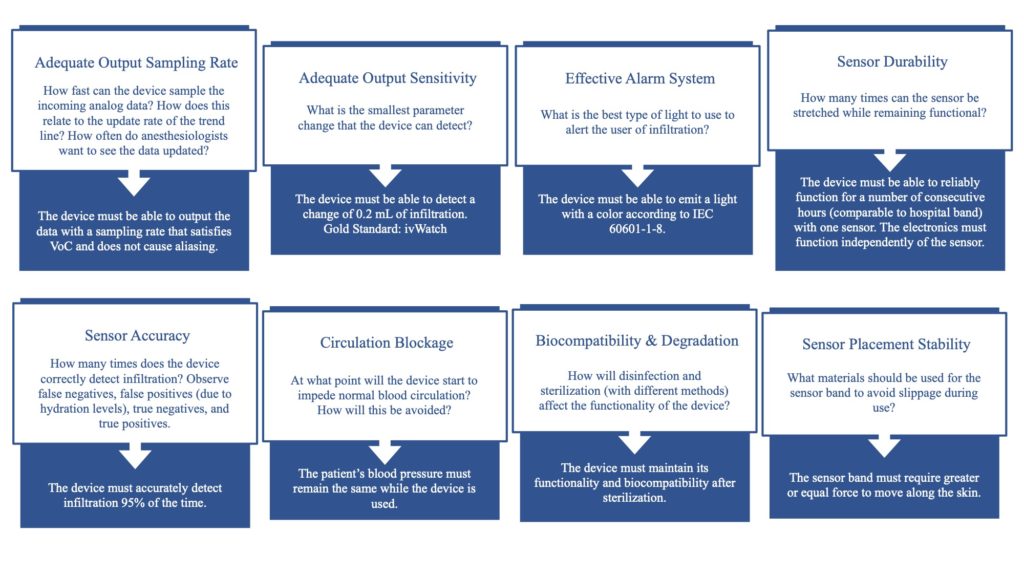
Risk Analysis
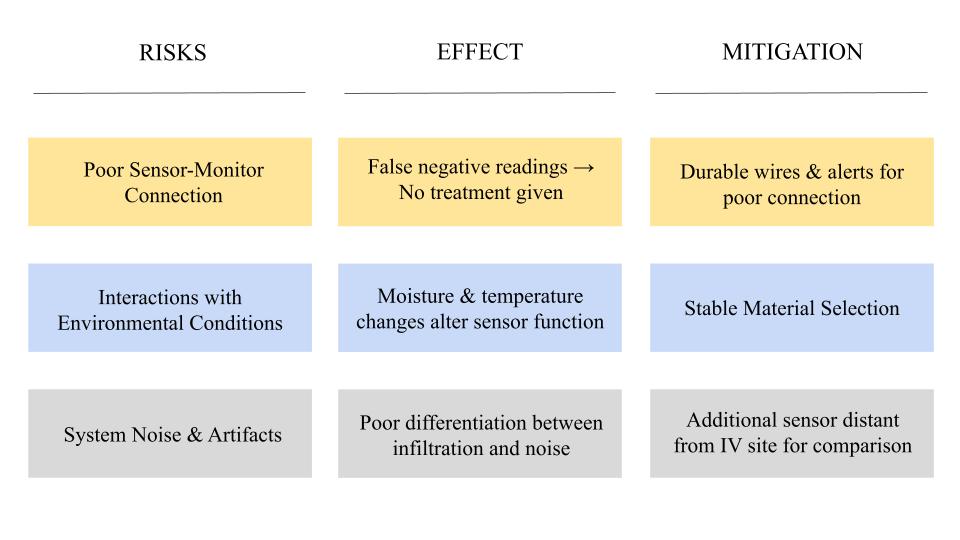
Heuristics & Human Factors
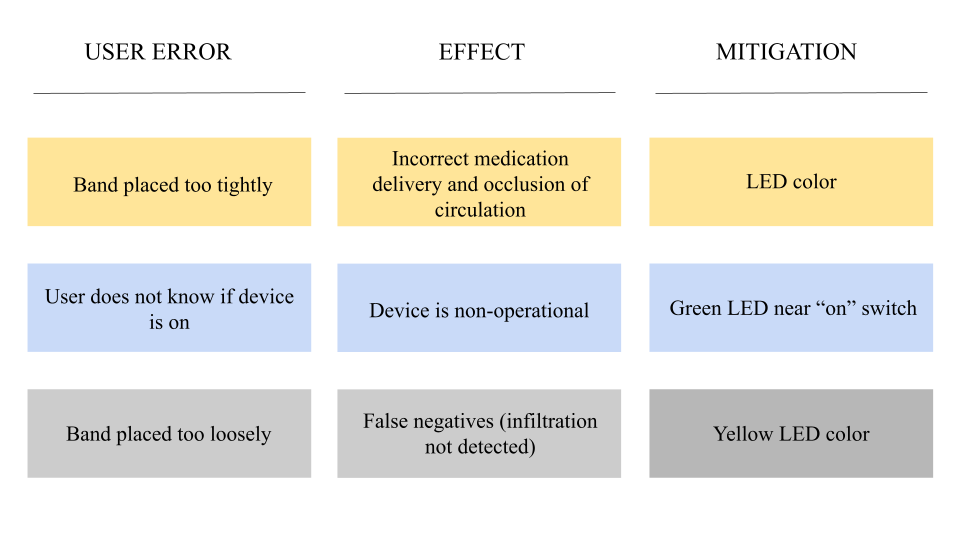
Intellectual Property Plan
FDA Teleconference Highlights
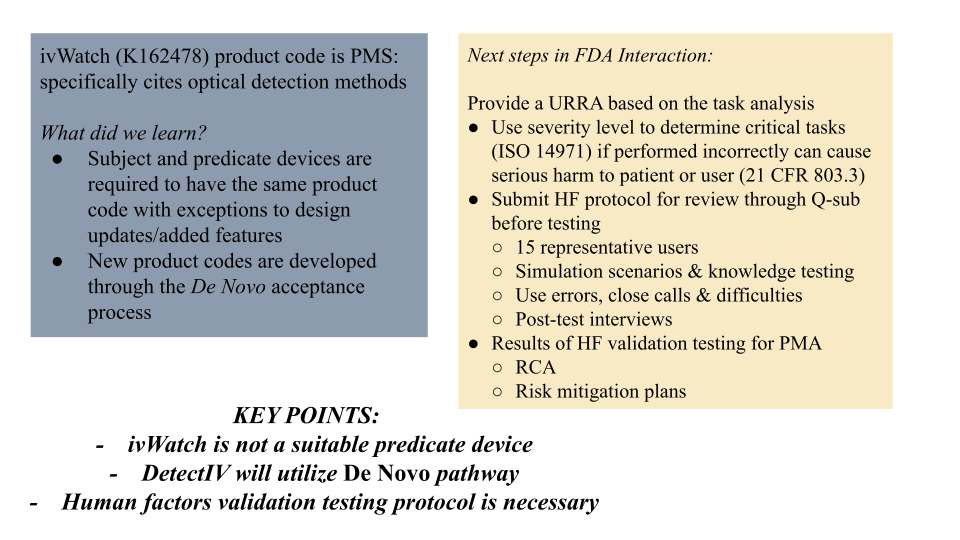
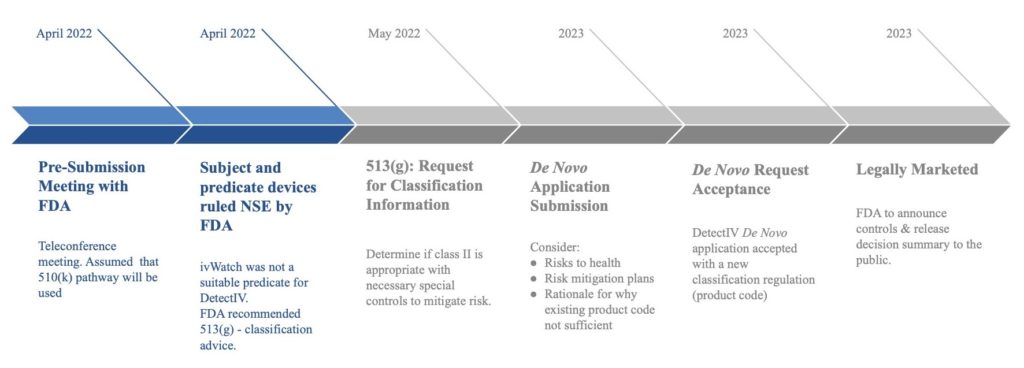
FDA Regulatory Plan
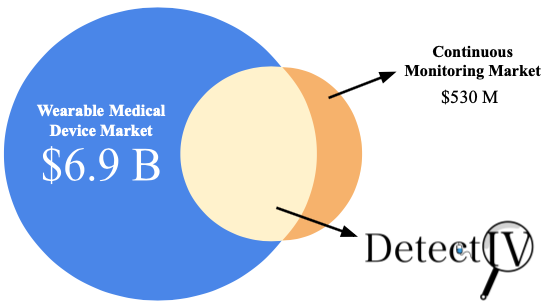
Market Opportunity
Goal: Enter a segment of the wearable and continuous monitoring device markets.
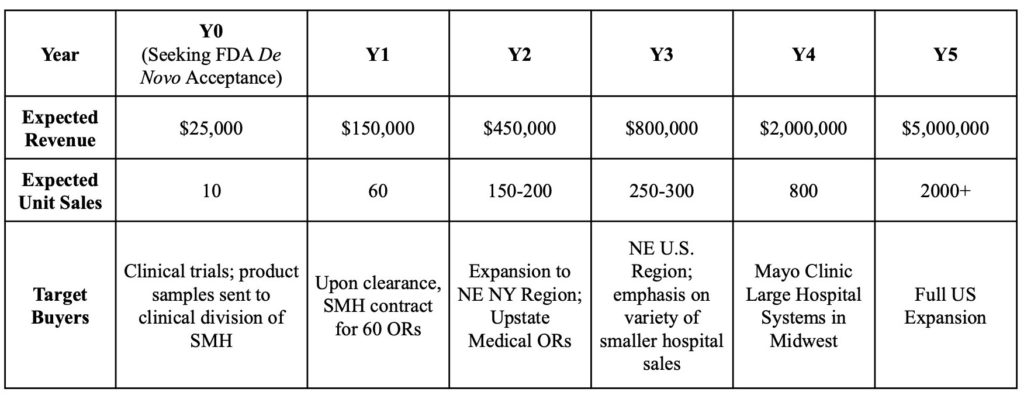
Business Plan
Value propositions include anesthesiologists relying on the DetectIV to detect infiltration when the IV insertion site is not visible or accessible. Key activities include purchasing materials, manufacturing the device, verification, and validation of the device through different types of testing, and FDA De Novo acceptance (DetectIV will most likely be Class II). Key activity expenses include the De Novo submission fee ($5,600 annual establishment fee + $28,114 De Novo submission), material purchase, shipment, distribution shipment, packaging, and labor costs. DetectIV is seeking $300,000 series A funding to reach the milestone of FDA De Novo acceptance. Revenue streams include hospital purchasing teams that will pay a unit price for each device through the channel of the device manufacturer to the hospital purchasing storage.
Reimbursement Strategy
Acknowledgments

References
[1]“Products,” ivWatch, 12-May-2021. [Online]. Available: https://www.ivwatch.com/products/.
[2] “Causes of IV Infiltration – Training Video,” www.youtube.com. https://www.youtube.com/watch?v=pLonu3XeV3I.
[3] Kalorama Information, October 2016. [Online from marketresearch.com]. [Accessed 15 March 2022].
[4] Wearable Medical Device Market Size Report, 2030. [Online]. Available: https://www.grandviewresearch.com/industry-analysis/wearable-medical-devices-market. [Accessed: 17-Apr-2022].
Contact Us
Sophie Mackenzie
Theo Couderc
Rebecca Mathurin
