We have developed a neonatal intubation probe that utilizes bioimpedance technology to accurately verify the placement of the endotracheal tube. This device aims to reduce the risk of misplacement during intubation, enhancing patient safety and outcomes. By providing feedback to healthcare providers, our device facilitates efficient and precise intubation in neonates, ultimately improving clinical care and reducing complications.
Problem Statement
ETTs can become dislodged and repositioned in the esophagus, leading to complications such as hypoxia, respiratory failure, cardiac arrest, brain damage, or mortality [2]. Currently, the only way to validate ETT placement in neonates is direct visualization with a laryngoscope, necessitating a less invasive approach [1].
Background
Neonatal tracheal intubation is a life saving procedure that is performed during respiratory failure, apnea, surgical procedures, or upsizing of an existing endotracheal tube. Given the small size of neonates, this procedure can potentially be dangerous if proper visualization of the patients’ trachea cannot be obtained by the provider [1]. Failure to intubate a patients’ airway in a timely manner may lead to complications such as hypoxia, respiratory failure, cardiac arrest, brain damage, or mortality [2]. Research has shown that as the number of intubation attempts increases, the neonate’s risk of intraventricular hemorrhage (IVH) increases. IVH, or bleeding into the ventricles surrounding the brain, may have a variety of impact on newborns, ranging from similar outcomes as premature babies to severe developmental delays and movement problems [2]. To reduce the time needed to intubate neonates and prevent the need for multiple intubation attempts, we plan to design an endotracheal intubation device that validates proper placement within the patient’s trachea without needing to hyperextend the patient’s neck to gain visualization of the airway.
Design Metrics
| Metric | Goal |
| Time of intubation | < 30 seconds |
| Degree of neck rotation | 0 degrees |
| Sterility assurance level | < 10e-6 |
| Current | < 10 µA |
| Tube diameter | 2-12mm in .5mm increments |
Description of Procedure
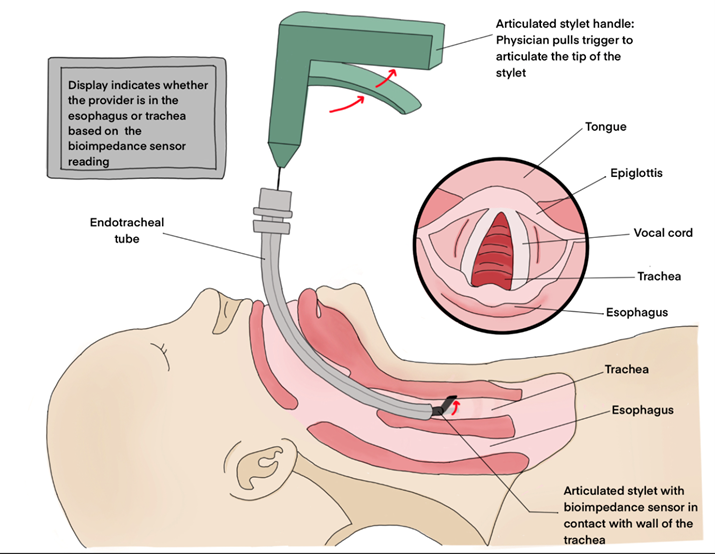
This procedure aims to verify the placement of an endotracheal tube that has already been inserted into a neonate via intubation. The provider will simply insert the stylet down the endotracheal tube, and squeeze the handle to activate the bioimpedance reader. The sensor at the tip of the stylet will read the impedance of the tissue wall, and notify the provider if it is respiratory tissue or esophageal tissue.
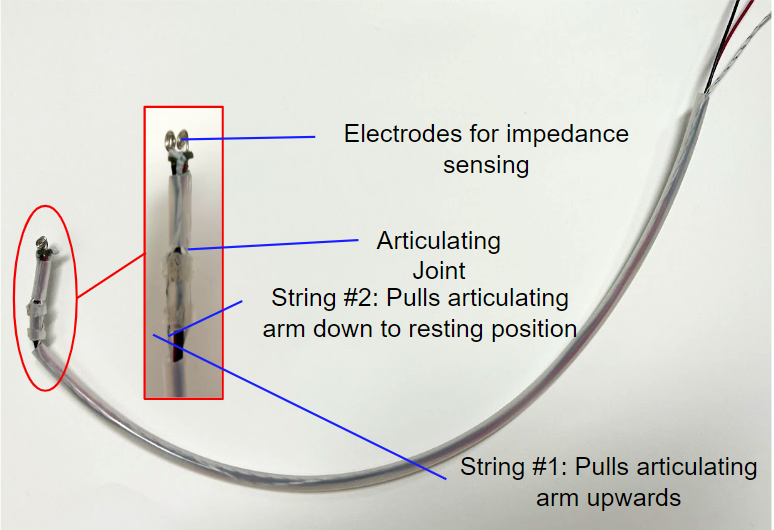
Articulating Stylet Prototype
The articulating stylet provides a mechanism for the sensor electrodes to make contact with the tracheal and esophageal tissues.
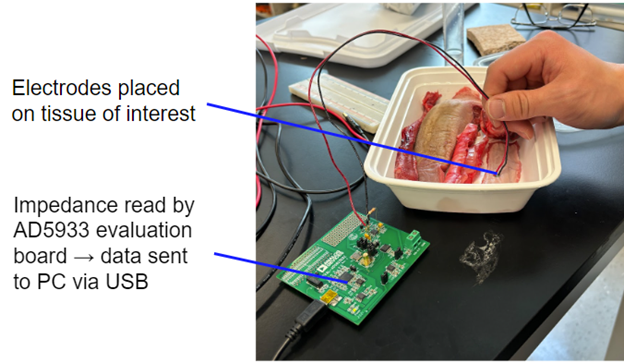
Bioimpedance Testing
Tissues measured for bioimpedance:
- Porcine tracheal lining
- Porcine esophageal lining
- Porcine posterior lingual surface
- Physiological buffer (control)
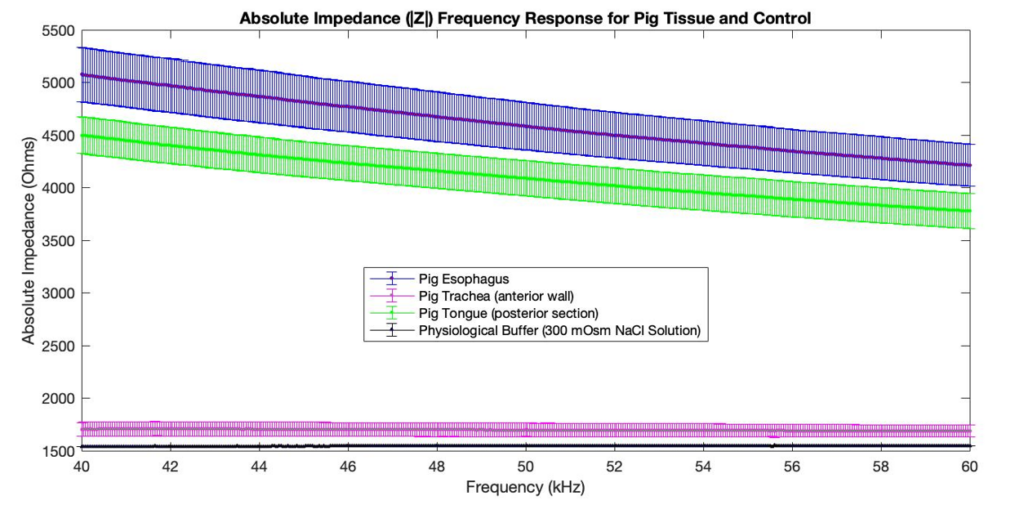
Bioimpedance Sensor Data
The following can be concluded based on the results:
- The pig esophagus impedance profile has a higher magnitude than the pig trachea impedance profile.
- The pig esophagus impedance profile is frequency dependent in the 40-60 kHz range, whereas the pig trachea impedance profile is not.
- The pig tongue impedance profile is similar to the pig esophagus impedance profile (both are muscular structures).
Conclusions & Future Directions
- Bioimpedance measurements can be used to reliably distinguish between tracheal and endotracheal tissue
- Next step #1: Miniaturization of stylet and refinement of articulating mechanism to enable compatibility with neonatal ETTs
- Next step #2: Development of small-scale custom electronics with an intuitive feedback system to make the device fully portable and user-friendly
Acknowledgements
Thanks to Dr. Brown for the logistical guidance he has provided since the start of this project, Dr. Seidman for helping with the acquisition of our bioimpedance sensing circuit and pig tissue for testing , and Dr. Barbut (our customer) for supplying endotracheal tubes and facilitating a comprehensive NICU tour.
Team Members
Hannah Bushey, Elizabeth McGinn, Laura Nafis, Benedikt Winzer, Thomas Xue
Customer
Dr. Gal Barbut, M.D., Department of Pediatrics, Neonatology, UR Medical Center
Supervisor
Dr. Edward Brown, Ph. D, Biomedical Engineering, Neuroscience, James Wilmot Cancer Center, University of Rochester
