
Bubble Trouble is the industry’s first and only medical device company that aims to eliminate bubbles in all surgical procedures to aid healthcare professionals prepare contrast agents faster, safer and simpler.
Bubble Trouble Mission Statement
The Team
| Michael C. Lee | Samuel C. Maquet |
| CEO & Co-Founder (UR BME ’21, CMTI ’22) | COO & Co-Founder (TCNJ BME ’21, CMTI ’22) |
Equipped with wholehearted passion, trust and a strong foundation, our team is eager to apply our biomedical engineering background to the clinical field to make a positive impact within the healthcare system.
Abstract
Bubble Trouble seeks to improve patient quality of care and operating room turnover. Bubble Trouble’s novel technology, Bubble Stopper, efficiently transports sterile fluids to aid clinicians in preparing contrast agent free from dangerous bubbles twice as fast compared to current practice. Its fully automated design enables clinicians to focus on more demanding tasks and provide better care to patients. The decreased need for contrast agent preparation will lead to a decrease in surgical time and improved patient care, resulting in an additional $3.6 million in annual revenue per operating room. Our technology has the potential to be the new standard of care in any procedure requiring fluids to be injected into patients such as for hemodialysis, angiograms, MRIs and infusions.


Problem
Hospitals hire technicians to fill syringes with contrast agent trying to avoid bubbles from forming – the slowest step for angiograms. Technicians must vigorously tap syringes to isolate and remove bubbles, prolonging the procedure, increasing tension among staff and patient anxiety. Approximately, 78 million computed tomography (CT) examinations are performed annually in the United States using contrast agents. Air embolization occurs at a rate of 15%, however as air bubbles can go unnoticed and are not easily distinguishable, the actual rate is likely higher. If left unchecked, these air embolisms can cascade and lead to prolonged stays in the hospital Figure 3.

Surgeons strive to improve surgery turnover to treat more patients, and provide better care for patients and more revenue for the hospital. Currently, it takes about 15 minutes to prepare bubble-free contrast agent in an injector syringe Figure 4. Extra time is needed throughout the surgery when clinicians exhaust all the prepared contrast agent and need more. During which all members in the operating room have to stop and wait until the additional bubble-free syringe of contrast agent is prepared and ready for use.

Current Practice

Interventional Radiology (IR) technicians and nurses have to be present in the operating room an hour before angiogram procedures. The longest step is preparing bubble-free contrast agent syringes (~15 min), which vary widely depending on the experience level of technicians. As portrayed above, the current process of filling the injector syringe is complicated, inconsistent and time-consuming Figure 5. Bubbles appear due to the negative pressure induced by the injector leading to cavitation, or the formation of bubbles when the pressure of liquid decreases below the liquid’s vapor pressure. The only preventative measure for bubbles from entering the patients is vigorously tapping the injector syringe Figure 6 to isolate visible bubbles, however, it is entirely dependent on the human eye. Repeated tapping caused multiple injuries to technicians and increased the burden for the technicians.
Voice of Customer
We have had the opportunity to pitch our idea and product to several neurosurgeons, medical students, leading medical device sales representatives, OR technicians and residents. We have received feedback from over fifteen subject matter experts regarding the clinical need, usability and other considerations for our device.
“It is a huge concern; bubbles may lead to air embolisms or in some cases a stroke. Surgeons and other clinicians are all aware of the problem. They all are nervous and cautious when preparing for angiogram procedures. I cannot agree more that there are catastrophic circumstances if the bubbles were to be neglected. Any possible action must be made to mitigate bubbles.”
– Emma Thompson; URMC IR Technician
“Bubbles are definitely bad for the patient’s well-being. There have been cases where patients died or experienced a stroke.”
– Dr. Jonathan Stone; URMC Neurosurgeon
Our Product: Bubble Stopper
The bubble removal process is comprised of a pump, tubing and our novel Bubble Stopper, utilizing positive pressure to deliver contrast agents to the injector syringe. With just one click, our device fills the syringes used in angiograms faster, safer and more reliably. This allows the technician to perform other key tasks to speed up the clinical workflow. The process for users to utilize our device is outlined in Figure 2.
Below is an animation that describes the concept behind the device. It also describes one of the possible directions we may take in the future, which is to add a sensor to improve the automation and functionality of our system Figure 6.
Finally, the following figure highlights the major prototypes that we used for testing. Going through iterations and making design changes, we were able to learn from each prototype generation. The final prototype consists of ideal dimensions for the most commonly used injector syringes Figure 8.

Product: Exceed Expectations
Staying in accordance with Bubble Trouble’s goal, Bubble Stopper is designed to reduce the time it takes to fill an injector syringe with bubble-free contrast agent. Number of design and usability tests were conducted to evaluate the functionality of our device. All of which showed promising results to move forward with our prototype development. Here, two critical test results that exceeded expectations are shown. The following figure shows a block diagram that compares the current practice and Bubble Trouble method of preparing a contrast agent before an angiogram procedure Figure 9.

Design of Experiment (DOE)
Knowing that the time to fill a syringe injector with current practice takes a minimum of 10 minutes, we aimed our device to cut the preparation time by half. One of the design specifications of our device was that it had to fill the syringe injector 5 minutes. We utilized design of experiments (DOE) to the find the optimal tube diameter, angle and flow rate that minimizes the fill time (Figure 10).

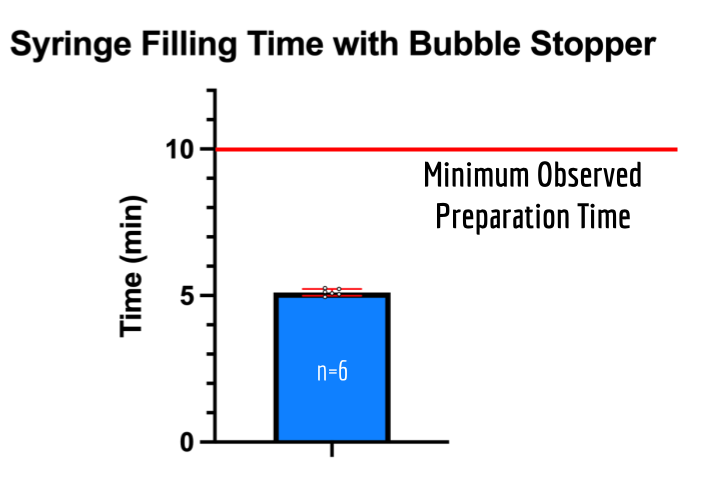
Test #1: Decrease in Preparation Time
From the results from our DOE, we utilized the most effective set-up to test for the time to fill the injector syringe with our Bubble Stopper. The following are the results from 6 trials (Figure 11). Results showed that the average time to fill an injector syringe with our system was 5.11 min, which is 48.9% decrease relative to the current minimum preparation time (p<0.01).

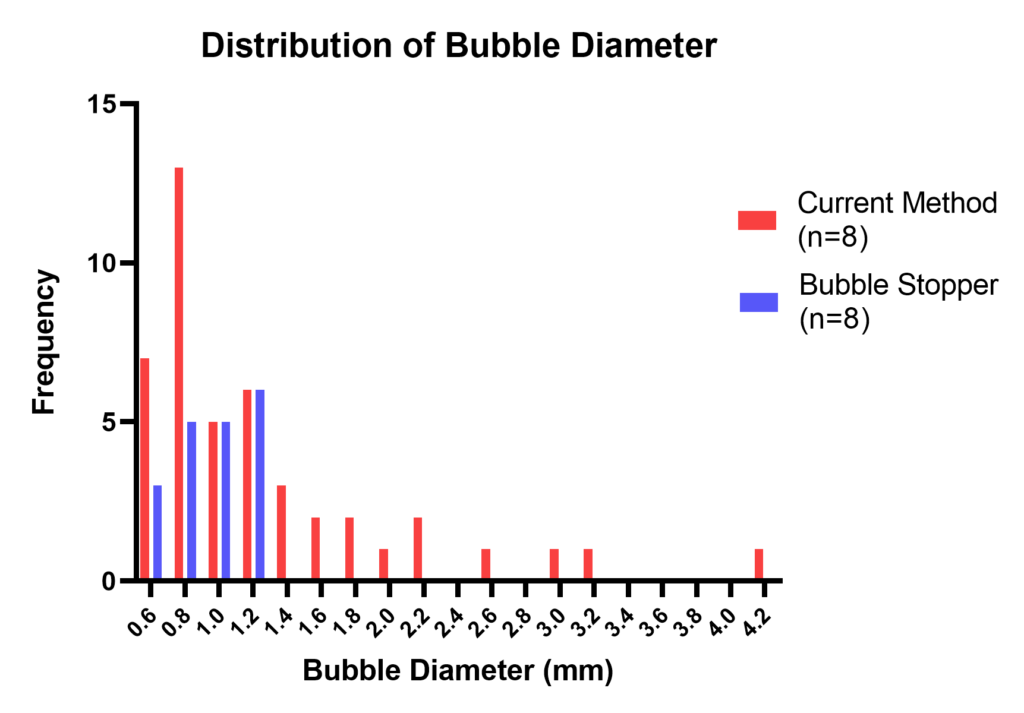
Test #2: Improvement in Functionality (Decrease in Bubble Size)
The second test focused on examining the functionality of our device. One of the design specifications of our device was that it had to significantly decrease the size and volume of air bubbles in the injector syringe. We used the same set-up as test #1 but analyzed the observed bubbles using ImageJ. The baseline for comparison was the bubbles observed in the injector syringes filled using current practice. The images for baseline analysis were provided by our IR technician collaborators.
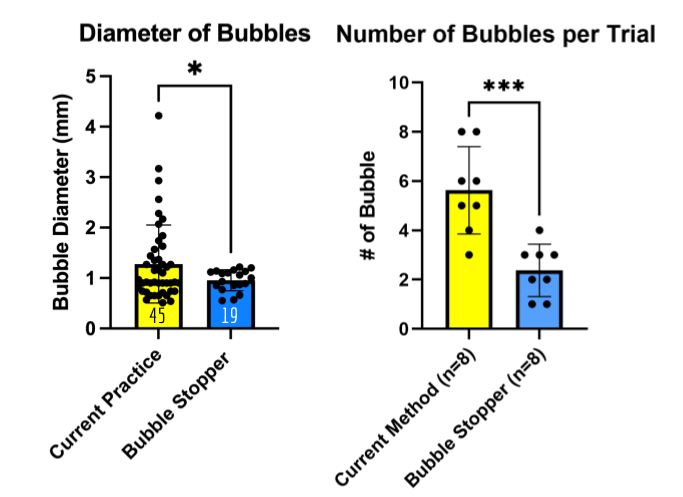
Results from 6 trials showed that there was a statistically significant decrease in bubble size and quantity with our device (p<0.05). There is a 58% decrease in the quantity of bubbles and 25% decrease in the bubble diameter. The number of bubbles emerging in each trial has decreased by two-fold. (Figure 12).
Diameter of bubbles measured using ImageJ, analyzed distribution using F-test (JMP, Prism). Variances between two groups are not equal (p<0.0001, F-Ratio = 13.7149). Bubbles formed using the current method are bigger and more variable in size.
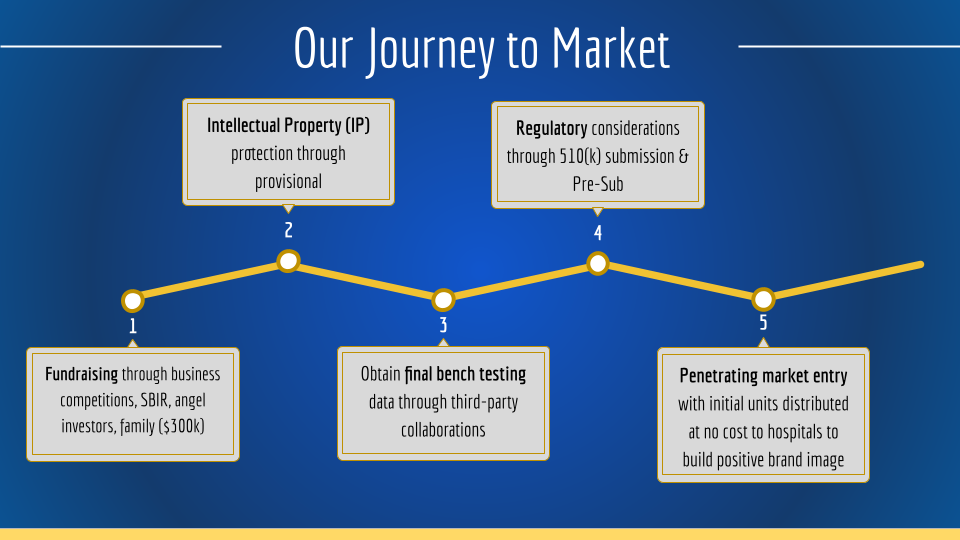
Our Path to Market
- Finalize prototype while maintaining strict documentation of design changes and adhering to appropriate standards: ISO13485, ISO10993-1, ANSI/AAMI HE75, 21 CFR 820 and more.
- Obtain funding by pitching to angel investors, SBIR grants and business competition.
- File a provisional patent application for IP protection.
- Work with a Contract Research Organization (CRO) and obtain bench testing data for the final prototype.
- Utilize the safety data for a 510(k) submission for clearance with the FDA while also leveraging the pre-submission program.
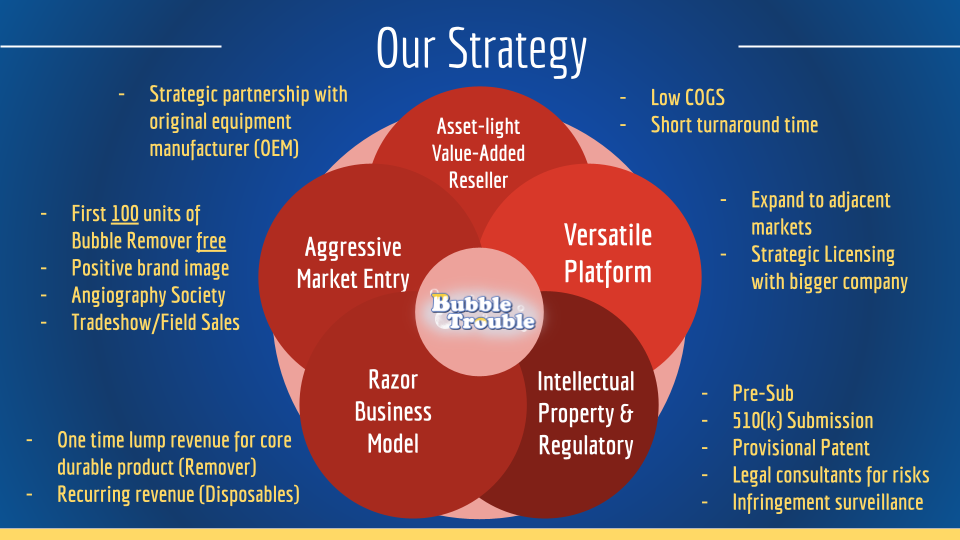
- Deliver the first 100 units to hospitals across upstate New York for free to create a positive brand image with OR staff to convince hospital purchasing committees of the utility of our platform.
Target Market
The global angiography equipment market size in 2022 is worth $10.87 billion and is expected to grow at a CAGR of 5.12% until 2027. The global autoinjectors comprise a market segment worth $46 billion in 2020 and are expected to grow to $104.9 billion by 2025. Expanding into the hemodialysis market would add an additional target market worth $71.5 billion with a CAGR growth rate of 4.2%.
On top of the current market, we expect to create a new market for technology focusing on optimizing surgical preparation and improving surgical turnover time. The following is an estimate of the total revenue hospitals are going to raise annually after implementing our device. Saving an average of an hour per operating room per day, the following figures consider the case where an additional angiogram procedure is done with the saved time. Additional revenue can vary as clinicians can use the time for other procedures Figure 14.
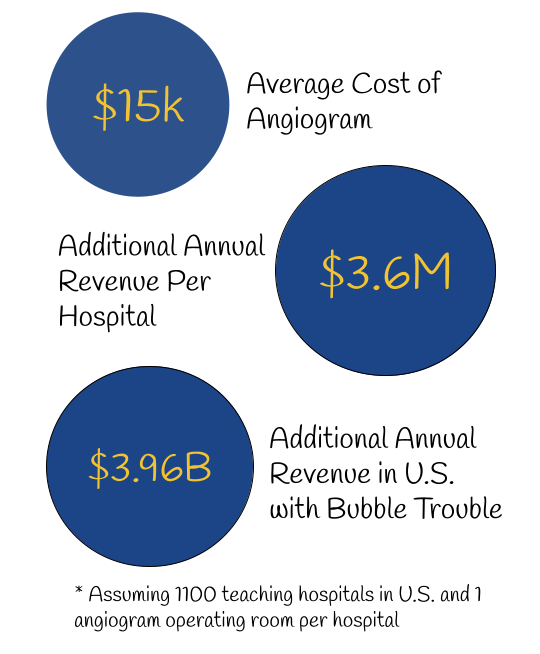
Competitive Advantage
There are no comparable devices that aim to transport sterile fluids in a quick, safe, simple, and cost-effective manner. However, potential competitors are major auto-injector companies such as GE Healthcare, Bayer, and Bracco. There may be efforts in their research pipeline to address the issue of potential dangers of visible bubbles in their injector syringes and the resulting delay in angiogram procedures. Other competition may emerge after our initial launch, which will be deterred through provisional patent applications and constant IP monitoring.
Our device will be the first of its kind to receive FDA clearance. Thanks to early IP protection efforts, Bubble Trouble will be the leader in eliminating dangerous bubbles while saving time and increasing surgery throughput. Our product does not disrupt the current standard of care and is an easy-to-implement solution. There is no need for prior training to use our device. Our device has undergone in-depth human factors analysis and checks for quality to develop workable and effective countermeasures to potential adverse events. Finally, we are competitively priced. Average costs for contrast agent injectors are $10,000, and consumables are $600. Bubble Trouble is an attractive, cost-effective addition to current surgical equipment that will generate additional revenue for the hospital.
Business: We Make Money
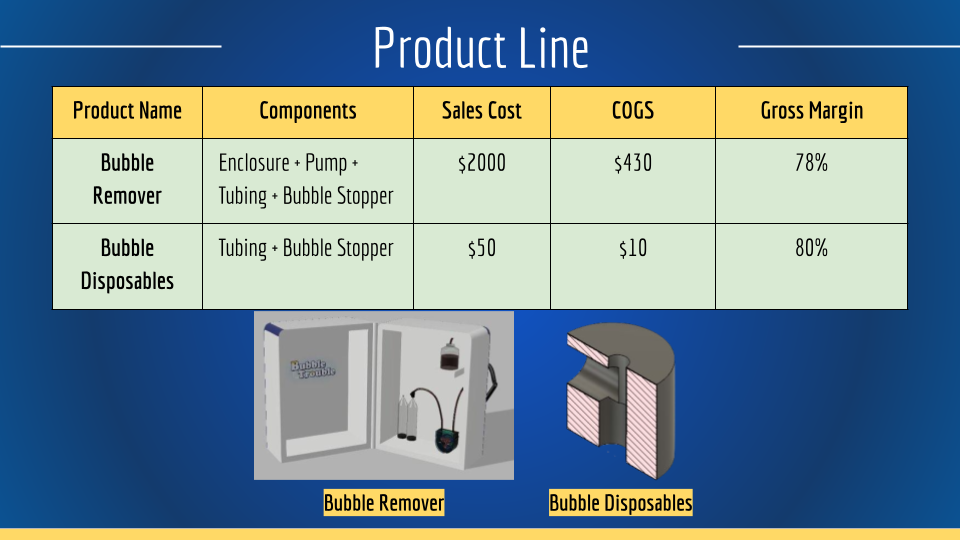
Bubble Trouble has two sources of revenue. The initial device purchase is followed by recurring purchases of single-use Bubble Stoppers and tubes. Each operating room needs one initial purchase of the full device and new sets of stoppers and tubing are needed for each angiogram procedure. For a strong market entry, we will give 100 units to hospitals across the US for free. This will create a positive brand image with hospital purchasing committees, invaluable to our efforts. Moving forward, the company will charge $2,000 for the entire device and $50 for the disposables, with future price adjustments.

Exit Strategy: Bubble Trouble will primarily pursue an exit strategy targeted toward licensing, expansion, and a favorable acquisition. We will license the technology once sufficiently mature to injector manufacturers such as GE Healthcare, Bayer, and Bracco. Our term sheet would maintain internal ownership of the intellectual property, allowing us to expand into adjacent markets such as hemodialysis, MRIs and infusions. This strategy allows us to stay in operation and wait for the highest acquisition price.
Future Direction
With continuous support and positive feedback from potential customers and users, Bubble Trouble is always striving to improve our device. At this point, we have three major goals that seem promising and will improve our product. These goals were determined by considering the results from our tests, the voice of customer feedback, conversations with experts in the field, human factor analysis, and quality system evaluations.
I. Build an all-in-one enclosure system to put compartments into one space. This enclosure will make our system less disruptive, less bulky, more convenient, and time-efficient (Figure 17).
II. Incorporate micropores and microfiltration membranes in the tubing to further eliminate bubbles. The addition of membranes will lead to improvement in bubble elimination and further reduce the rate of complications. It also opens the possibility of real-time filtering during the procedure.
III. Add an ultrasound sensor at the exit port to further check for residual bubbles left in the system. The sensor will provide more confidence to the clinicians and enable us to better quantify air in the system.

Mentors & Acknowledgement
- Greg Gdowski (Executive Director of CMTI)
- Marty Gira (Senior Research Engineer)
- Amy Lerner (Professor of Biomedical Engineering)
- Dr. Jonathan Stone (Neurosurgeon)
- Dr. Tarun Bhalla (Neurosurgeon)
- James McGrath (Professor of Biomedical Engineering)
- Diane Dalecki (Department Chair of Biomedical Engineering)
- Mahllet Beyene (CMTI Graduate Program Coordinator)
- Emma Thompson (IR Technician)
- Brianna Stelmaszky (Radiation Technician)
- Kathryn Willison (Radiation Technician)
- June Kim (Collaborator)
- wholehearted thanks to all colleagues, collaborators & clinicians

Thank you everyone for visiting our website and providing interest in our project! We look forward to making a positive impact on the healthcare field. It will be our pleasure and honor to talk to you further about our project. Please comment below or send us an email at OfficialBubbleTrouble@gmail.com.


References
[1] “MEDRAD Mark 7 Arterion | Radiology”, Manuals.radiology.bayer.com, 2022. [Online]. Available: https://manuals.radiology.bayer.com/products/medrad-mark-7-arterion.
[2] C. M. Muth and E. S. Shank, “Gas Embolism,” New England Journal of Medicine, vol. 342, no. 7, pp. 476-482, 2000
[3] Childers CP, Maggard-Gibbons M. Understanding costs of care in the operating room. [published online February 28, 2018] AMA Surg. doi:10.1001/jamasurg.2017.6233

