The Steady-State Excess Sodium Sulfite Method
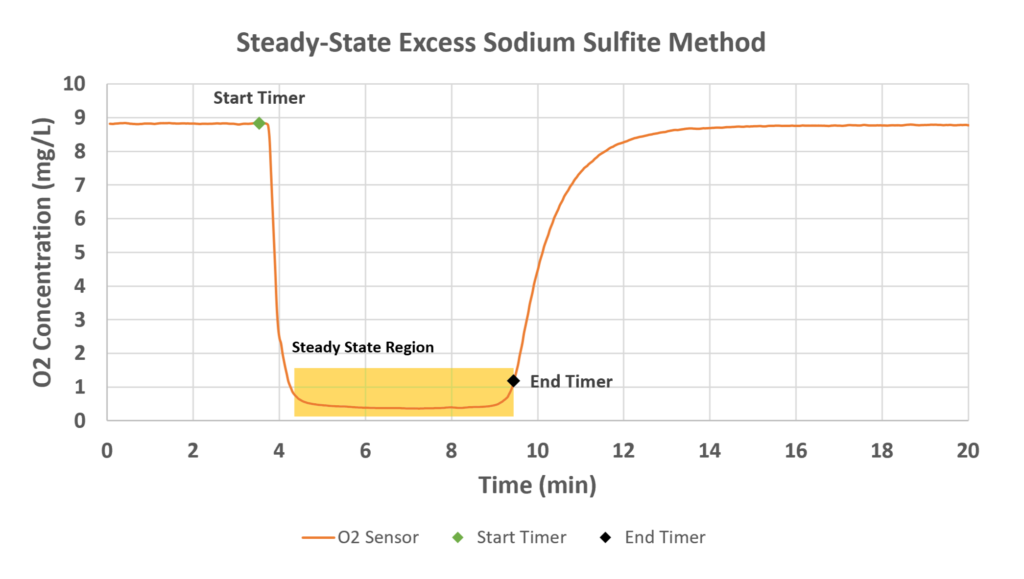
The steady-state excess sodium sulfite method used by SPX FLOW to characterize the OTR is represented in Equation 1. It begins with aerating a tank of water in the presence of a cobalt chloride catalyst (CoCl2·6H2O). When the sodium sulfite is added, the O2 concentration rapidly decreases until it reaches a steady minimum. This is where the OTR is assumed to be constant.
2Na2 SO3+O2 → 2Na2 SO4 (Equation 1)
SPX FLOW aims to characterize the OTR in this reaction in fluids more viscous than water. In 2021, University of Rochester CHE Senior Design Team Whomping Willow determined that polyethylene glycol (PEG) would modify the working fluid’s viscosity without interfering with this reaction. Since O2 sensors are incompatible with PEG, Team Chocolate hypothesized that the OTR could be achieved by following the other reactant in this equation: sodium sulfite.
Sodium Sulfite Mass Balance
A mass balance on the system allowed Equation 2 to be determined:
d[O2]/dt = kLA([O2] – [O2*]) – krxn[Na2SO3]2[O2] (Equation 2)
In the steady-state region, d[O2]/dt = 0, so here the OTR can be calculated using the change in the concentration of sulfite over time:
OTR = (-1/2)(d[SO32- ]/dt) ≅ (-1/2)(∆[SO32-]/∆t) (Equation 3)
Basis of Design
Team Chocolate’s goal this semester was to design a way to continuously detect the concentration of sodium sulfite over the course of this reaction. This method would use Equation 3 to obtain the OTR with an associated confidence interval. In order to improve upon the O2 sensor’s capabilities, Team Chocolate’s design will also have to be compatible with the viscosity modifier, PEG.
It was hypothesized with the help of Professor Doug Kelley that the sulfite concentration could be detected by means of light absorbance. Team Whomping Willow had previously determined with high-performance liquid chromatography (HPLC) that in aqueous solution, sodium sulfite absorbed around a 275 nm wavelength. Team Chocolate then began work to make a UVC LED sensor with the following hypotheses:
| # | Hypothesis | Description | Testing Method |
| 1 | ABS ≠ f(PEG Concentration) | The absorbance of sulfite will not be a function of PEG concentration. | HPLC and UV-Vis |
| 2 | TR(Sulfite) ≅ 2 x OTR (Oxygen) | The sulfite transfer rate will be approximately twice the OTR. | Sulfite Strips and Test Kit |
| 3 | TR(Sulfite) = constant | The sulfite transfer rate will be constant at steady state. | Sulfite Strips and Test Kit |
| 4 | TR(Sulfite) = f(Viscosity) | The sulfite transfer rate will be a function of viscosity. | Sulfite Sensor and Test Kit |
