Team Members
- Lauren Redus
- Samuel Meil
- Autumn Kudlack
- Ana Cerezer
Project Sponsor
Dr. Wyatt Tenhaeff, Chemical Engineering
Abstract
Initiated Chemical Vapor Deposition (iCVD) is a technique utilized by Tenhaeff Labs at the University of Rochester to control the properties of polymeric thin films and coatings. A critical component of studying iCVD kinetics is dependent on the concentration of the monomer and initiator in the gas phase. This concentration measurement is nontrivial due to the low volatility of the monomers, and therefore, there is demonstrated need for the construction and implementation of a concentration measurement system.
Background
The monomer and initiator are vaporized using a bubbler system and co-fed into the iCVD reactor. Typical iCVD monomers are large organic molecules with low vapor pressures at room temperature, and as a result, are present at very low vapor concentrations. Therefore, measurement methods must be sensitive enough to identify these small composition changes with the mixture of the monomer and carrier gas.
System Requirements
- Operating conditions of 1 atm, 25°C
- Continuous concentration measurement system capable of being integrated into the current iCVD system used by Tenhaeff Labs
- Model compound with similar properties to butyl acrylate
- Optimize bubbler design
Proposed Approach
- Chosen Model Compound: ethyl acetate
- Infrared Radiation Cell (IR): IR LED emitter + IR receiver, chosen compound absorbs within C-H wavenumber of 3.4 µm
- Mass Flow Measurements (MFC/MFM): measure difference before and after bubbler system through a small differential heat transfer
- Gas Chromatograph (GC): correlate obtained voltages from IR cell with known concentrations
Results
Optimization Experiment
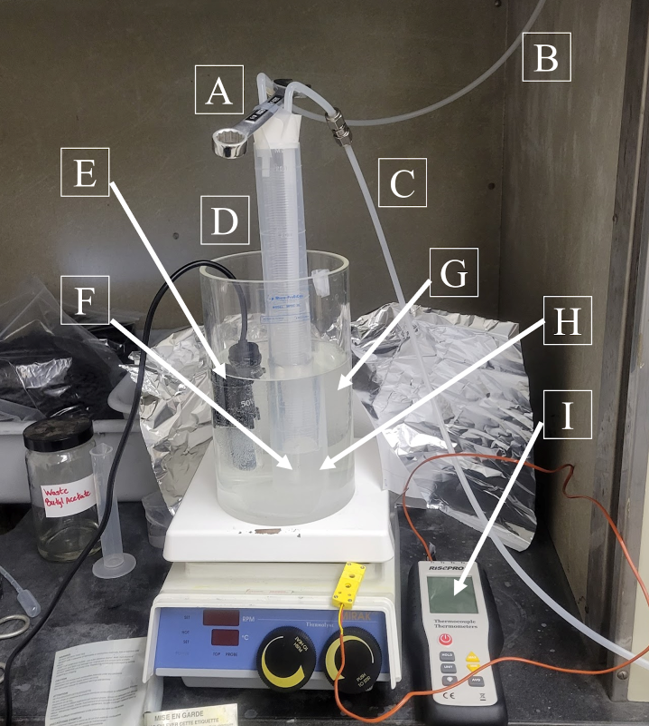
| Frit Type | Avg. Vaporization Rate (mL/h) | Flow Rate (SCCM) |
|---|---|---|
| Coarse | 3.0 | 13. ± 1.2 |
| Extra Fine | 2.8 | 12. ± 1.8 |
Mass Flow System
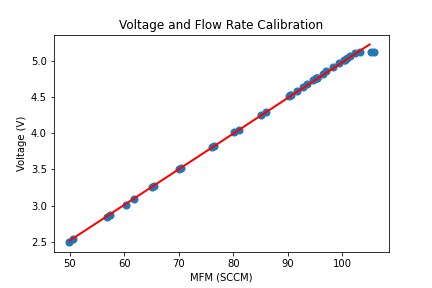
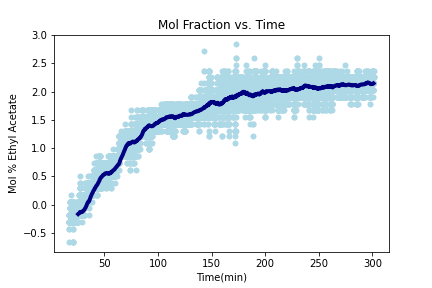
In 5 hours, ~ 2 mol% ethyl acetate was measured in the vapor phase.
Theoretical maximum ethyl acetate mole fraction percentage: 12 mol%
IR Cell
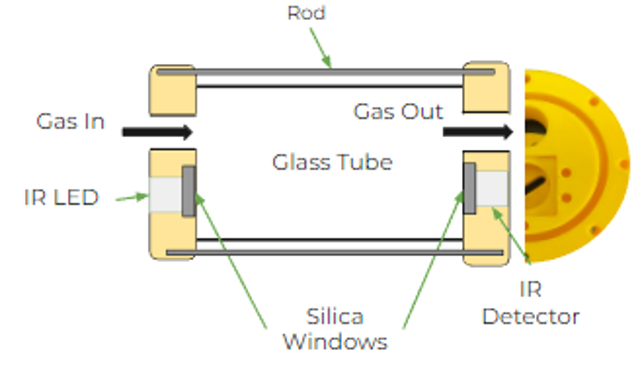

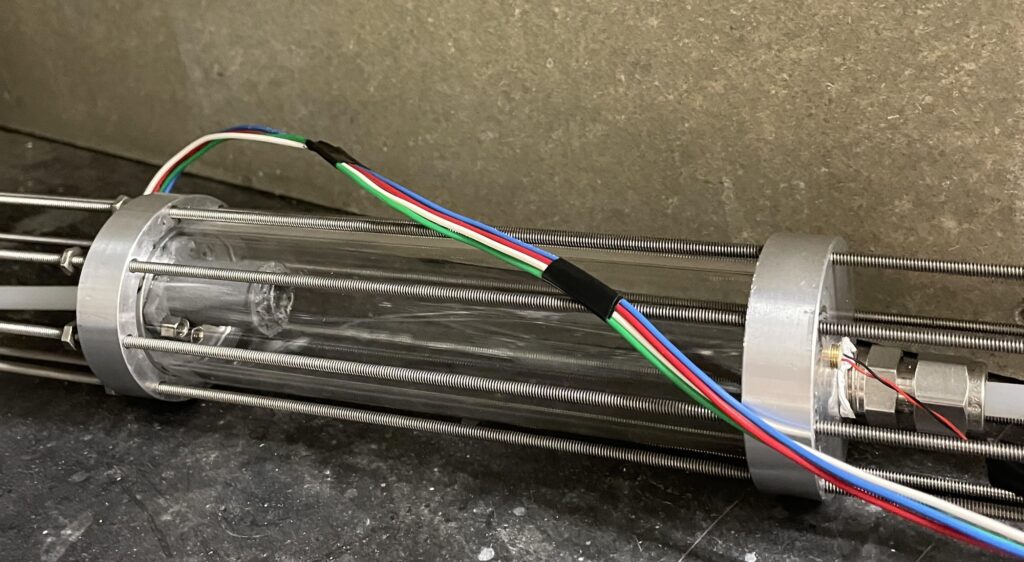
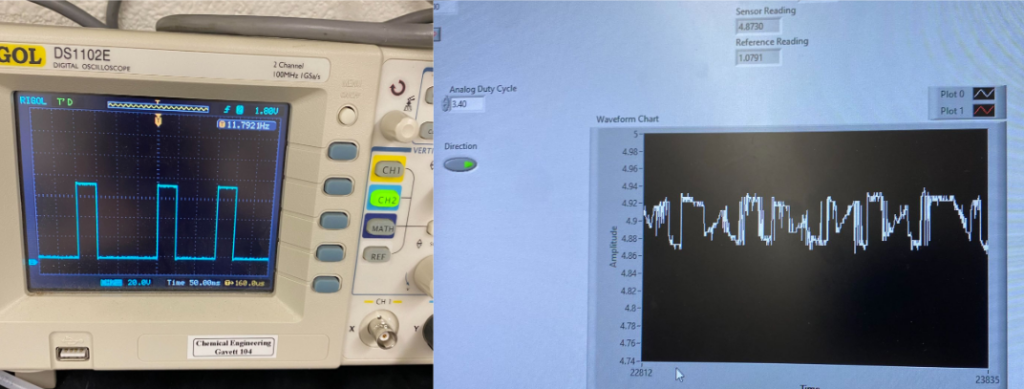
The IR cell utilizes an IR LED emitter and an IR sensor that operate with a wavenumber of 3.4 µm, the region where C-H absorbs in ethyl acetate. The measured absorbance can be related to concentration through Beer’s Law, where A = ɛcl. A circuit was implemented to enhance the signal from the sensor and operate with a pulsed IR light input.
Conclusion and Future Work
The differential measurements of the mass flow controllers displayed ~2 mol% ethyl acetate after 5 hours. The MFC system can be further improved with higher sensitivity and calibration of the instruments. The IR system is complete and additional testing is required to correlate the measured voltages with ethyl acetate concentration using a gas chromatograph.
Both prototype measurement methods demonstrate promise to be utilized within the Tenhaeff Labs and would require minimal changes to the current configuration of the iCVD reactors. The limiting factor for implementation of the proposed measurements is instrument sensitivity. If these methods are to be pursued further, it is suggested to build or utilize instrumentation that is more specialized and easily programmable. Vacuum conditions with these measurement methods should be explored as the current iCVD system is performed under vacuum pressure.
Acknowledgements
We would like to thank their project sponsor, Dr. Wyatt Tenhaeff, graduate students Fei Hu and Ni Huo, and their TA, Levi Sunday-Lefkowitz. Additionally, we would like to thank Dr. Doug Kelley, Clair Cunningham, Mason Garlatti, Dr. Melodie Lawton, Jeff Lefler, Professor Mark Juba, Wayne Griffin, and Paul Osborne. We are grateful for their time, support, and dedication to the project. This work was supported in part by the National Science Foundation under grant CBET-1845805.
