Purification of LUV Dye Using Column Chromatography
Quinton Dang, Waad Magram, Zhiwei Wang
Project Mentor
Mark Juba, University of Rochester
Project Sponsor
Brian Cleary, Kodak

Motivation
At Eastman Kodak, specialty chemicals, inks, and dispersions are manufactured for application in imaging products and photographic films.
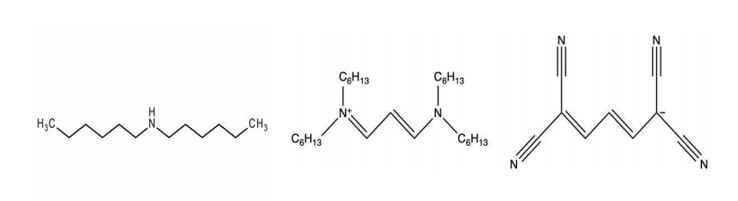
The LUV dye, [3-(dihexylamino)allylidene]malononitrile, is one such chemical that is used in the production of photographic film. Kodak manufactures crude LUV dye containing impurities that must be removed prior to being implemented into motion picture film.
In the crude LUV dye, there are two main impurities present. These impurities have boiling points that are both below and above the boiling point of the pure LUV dye. Specifically, the impurity dihexylamine has a boiling point of 192-195oC1. The other impurity, quat-propylamine, has a boiling point greater than 400.6oC. For pure LUV dye, the boiling point is 400.6oC at 1 atm2.
At Kodak, the current practice for the purification of pure LUV dye involves contracting a toll manufacturer to use a two-pass wiped-film evaporator to separate the impurities from the crude LUV dye. However, given the cost associated with contracting the toll manufacturer for the purification process, Kodak sought out the team to devise a lab-scale purification process which upon scale-up would be able to purify 2 kilograms of crude LUV dye per day.
