Team & Specialty:
We are Hemorrhage Heroes, one of the two teams focused on neurosurgical devices within the University of Rochester CMTI program. Our team is composed of Zachary Muench and Melisanda Feliz, both of who have degrees in Biomedical Engineering.


Background & Problem:
A cerebral aneurysm is a weakened portion of a blood vessel that bulges out and is filled with blood. These aneurysms currently affect around 6.5 million people and the US, and at least 16 million more are expected to develop one in their lifetime. Once an aneurysm develops, a person is at significant risk if it was to rupture, so aneurysms are treated in nearly all cases. The most established treatment method is surgical clipping of the aneurysm in which a small titanium clip is implanted at the base of the aneurysm in order to prevent blood from going into the aneurysm, and preventing it from growing further. The most significant risk of this procedure is the aneurysm rupturing while the surgeon is working. In the case of an intraoperative rupture, the patient can lose blood at an alarming rate, with liters of blood being lost in 10 minutes. This first obstructs the surgeon’s view, so they can no longer continue with the surgery until they regain control and a clear field of view. Additionally, the longer the patient continues to lose blood, the more likely it is the outcome of the surgery will be very poor, resulting in neurological deficits, significantly lengthened hospital stay, and increased costs.
The current method to correct an intraoperative ruptures involves multiple complicated maneuvers including the surgeon simultaneously removing the blood from the surgical cavity to restore their view, and placing a temporary aneurysm clip on the blood vessel to stop further bleeding while they continue the procedure, but this method is extremely difficult, and often takes minutes to complete successfully, all while the patient continues to lose blood and is at significant risk.
This leads to our biodesign statement which is as follows: “Develop a surgical device that minimizes blood loss and provides a clear surgical field to enhance outcomes and reduce complications during cerebral aneurysm clipping.”
The AneuAssist Device:
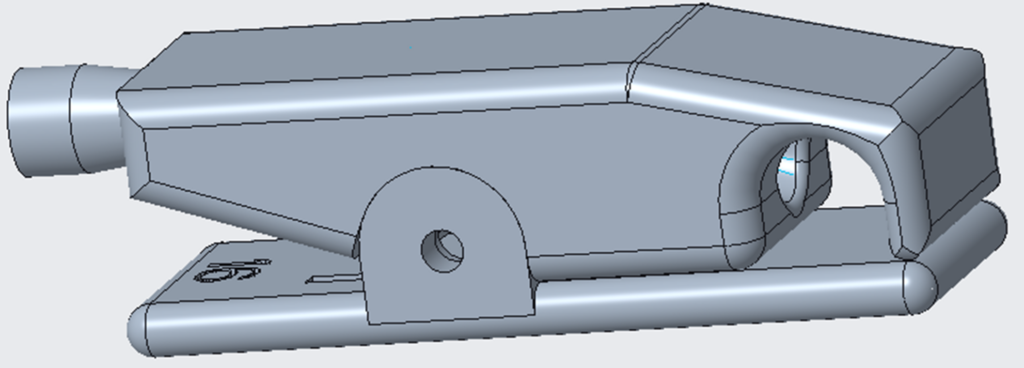
The AneuAssist device produced by Hemorrhage Heroes is the solution to the problem outlined above. The device is composed of a hard plastic clip that would be familiar to the surgeons using it, a spring and pivot to allow the device to open and close properly, and then a balloon, tube, and syringe which allow the surgeon to cut off the flow of blood. The clip is meant to be placed on the parent blood vessel before any other work is done on the aneurysm. Then, if the aneurysm ruptures, the surgeon can depress the plunger of the syringe which inflates the balloon and compresses the blood vessel. This provides the surgeon with fast, easy, and dynamic control of blood flow into the aneurysm, reducing the time needed to regain control after a rupture from minutes to a matter of seconds.
Testing:
To test the AneuAssist device, we created an analog for a blood vessel using latex tubing and a peristaltic pump. This set-up allowed us to simulate a use scenario at a larger scale, and accurately control selected parameters. The initial testing performed involved visually determining if the device successfully stopped the flow of water through the tube and pump set-up at various flow rates and levels of activation. The results were binary, either a yes or a no. To build on the results from the initial tests, we used a similar testing set-up but measured the volume of water that was collected during a set time period. In this second test, both the flow rate and level of activation for our device were varied again, allowing us to determine how well we achieved accurate dynamic control of the flow of water through the analog tubing and pump, as well as what the limits of the device were.
Market Opportunity:
The direct competition to our device are the temporary clips that are currently used. Because of this, the overall market size for our device is around $486.5 million USD. Our main market strategy would be to form partnerships with teaching hospitals, initially in the Rochester, Buffalo, and Syracuse regions, since they would be more likely to adopt newer technologies. In addition to this we would have a large presence within medical societies relating to neurosurgery such as the Neurosurgical Society of America, in which we could interact with a large number of surgeons who would be the end-user of our device, as well as partner with key opinion leaders who could help advertise and provide support for the AneuAssist device.
Regulatory Pathway:
Through in-depth conversations with the FDA, we determined that the most likely pathway for our device is through a De Novo Class II device classification process, in which we would compare our device to the existing aneurysm clips, as well as provide addition testing data in order to ensure the device is safe and effective.
Future Steps:
The next steps for the AneuAssist device is first to continue its development. The materials being used on the current proof-of-concept prototype would have to be refined, as well as the device would have to be reduced in size at least 10-fold. The final steps after product refinement would be patent applications to ensure the intellectual property surrounding the device is properly protected, and then FDA regulatory applications to ensure the device is cleared properly for marketing and sale.
