Dengue Virus Detection in Mosquitoes in Costa Rica
Authors

Ava Giorgianni
Jacob Kelley
Tiana Salomon
Kaelyn Schloss
Caroline Stockwell
Mentor
Dr. Edward Brown
Customers
Dr. Timothy Dye
Dr. Adriana Troyo Rodriguez
Dr. Benjamin Miller
Problem Statement
The JungleCup is a small, portable, and weather-proof device that can operate in various Costa Rican environments. This device attracts, traps, and kills female mosquitoes so that the number of mosquitoes carrying dengue virus can be evaluated to support health-related environmental research efforts.
The Device
The JungleCup is a one compartment device made from inexpensive, readily available materials. As shown in Figure 1, the device is made from an upside down, screw top Ziploc plastic container. The lactic-acid-soaked membrane, which is used to attract mosquitoes into the device and entice them to bite, rests on a petri dish inside the lid of the container. Sections of plastic on the walls of the device have been removed and replaced with mesh patches to allow for ventilation and detection of the lactic acid by mosquitoes outside of the cup. Mosquitoes are able to enter through the funnels that have been inserted into the mesh. This style of entry point has been used successfully in other bug traps to make entry easier and exiting more difficult. The black rain hoods cover the mesh portions of the cup to prevent rainfall from getting into the device, and the pink strings make it possible to hang the device at the desired length.
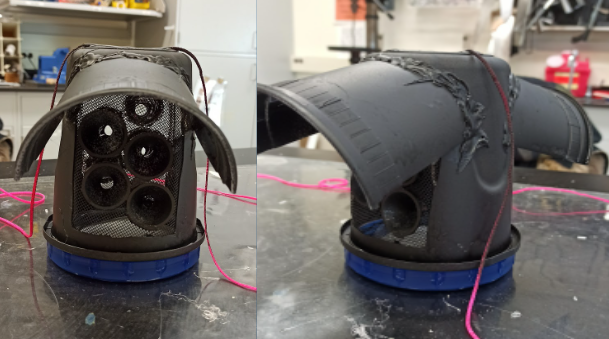
Figure 1: The JungleCup
Our customer plans to deploy around 20 of these devices near their laboratory in Costa Rica. In the field, each device will contain a pesticide strip to kill the mosquitoes that enter after about 5 minutes of exposure. The devices will be visited every 24-48 hours and the membranes in their petri dishes will be collected and replaced. The mosquito-bitten membranes will be taken back to the lab, where the lab members will conduct a colorimetric dot blot assay to analyze the membrane for the presence of dengue positive mosquito saliva.
The Analysis
After the membrane is brought back to the laboratory, colorimetric dot-blot assay will be performed. The assay used by the customer to detect dengue virus in the infected mosquitoes will use a biotinylated antibody specific for a dengue protein, non-structural protein 1 (NS1), to detect the viral protein. This detection will use a colorimetric readout by adding streptavidin HRP and a colorimetric TMB substrate. This reaction will result in blue dots on the membrane where the protein of interest was detected. In order to model the assay that the customer will use, ovalbumin was used as a model antigen to detect, as we were not able to work with dengue virus in the lab, and ovalbumin has a similar molecular weight to dengue NS1. The prototype we developed included spotting ovalbumin solution onto a PVDF membrane, blocking the membrane with BSA to take up the rest of the binding sites, washing the membrane then incubating it with a biotinylated anti-ovalbumin polyclonal antibody. Then, the membrane was washed again and steptavidin HRP was added and washed again. Finally, the TMB substrate was added to produce the colorimetric reaction.
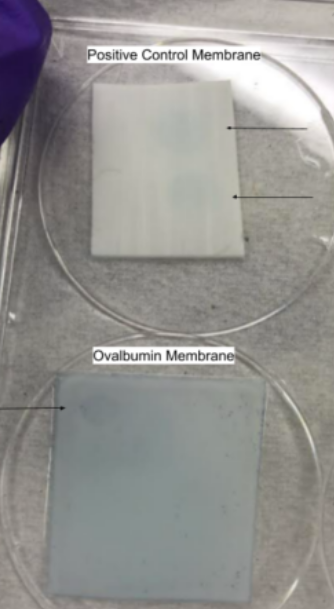
Figure 2: Colorimetric dot-blot assay results using ovalbumin
After performing the dot-blot assay, the images will be analyzed by a MATLAB Graphical User Interface (GUI) to quickly and efficiently quantify the number of dengue-positive dots.
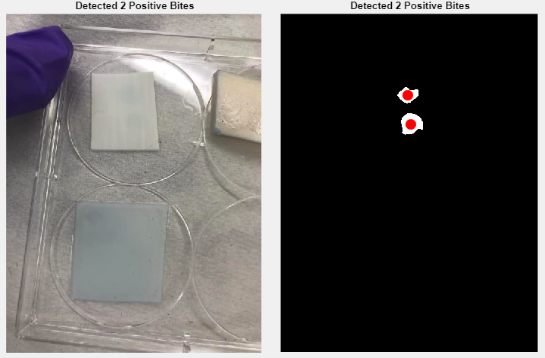
Figure 3: Result of MATLAB GUI
Future Directions
Currently there is a gap in knowledge where we were unable to test whether the mosquitoes will land on, and bite or probe the membrane thus leaving behind saliva and possibly dengue virus. This will need to be tested. Further, optimization of the colorimetric dot-blot assay will need to occur, and possibly a comparison to qPCR (the most sensitive detection method) to determine specificity of the assay as well. Finally, investigation into more sustainable and environmentally-friendly cup materials should be established.
Acknowledgements
TA: Allison Coon
Professors: Dr. Amy Lerner & Dr. Scott Seidman
Special thanks to:
Dr. Saravanan Thangamani (Upstate Medical University) for assisting us with mosquito testing of our device
Dr. James McGrath (University of Rochester) for providing guidance on the detection method
Dr. James Fry (University of Rochester) for assisting us with drosophila testing of our device
Dr. Michel Bryan (University of Rochester) for providing us insight on how to alter our detection method
Dr. Kanika Vats (University of Rochester) for providing us with ovalbumin
