Team and Specialty
We are REMA Tech, a team from the CMTI program at the University of Rochester who completed our clinical rotations in the neurosurgery department at Strong Memorial Hospital. Our team consists of Arjun Ashok, Emily Palacio, and Regina Yu.
Medical Condition
Our device will be used on patients undergoing a Kyphoplasty procedure, which is a minimally invasive treatment for vertebral compression fractures (VCFs). VCFs have a characteristic wedge-shaped deformity that is caused by the compromise to the anterior column of the spine [1]. These types of fractures affect about 1.5 million people per year in the United States alone, and of those about one third of them get clinically diagnosed and treated. VCFs are typically more common in older individuals with osteoporosis, but can affect a younger population if they have undergone a traumatic event [1].
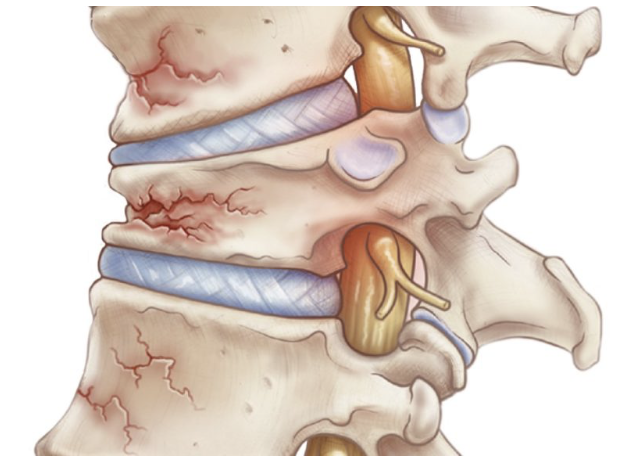
Surgical Difficulty/Problem
At Strong Memorial Hospital, a bone biopsy is taken every time a kyphoplasty is performed. The problem clinicians run into when taking a biopsy is they don’t know if they have retrieved an adequately sized sample, until after the needle is removed from the patient. There are times when an insufficient amount is collected and other times when nothing is collected at all, meaning at some point the sample breaks off or leaks out of the needle. The lack of sufficient sample means the needle may need to be inserted multiple times, increasing the risk to the patient and potentially leading to a misdiagnosis.
Market Opportunity
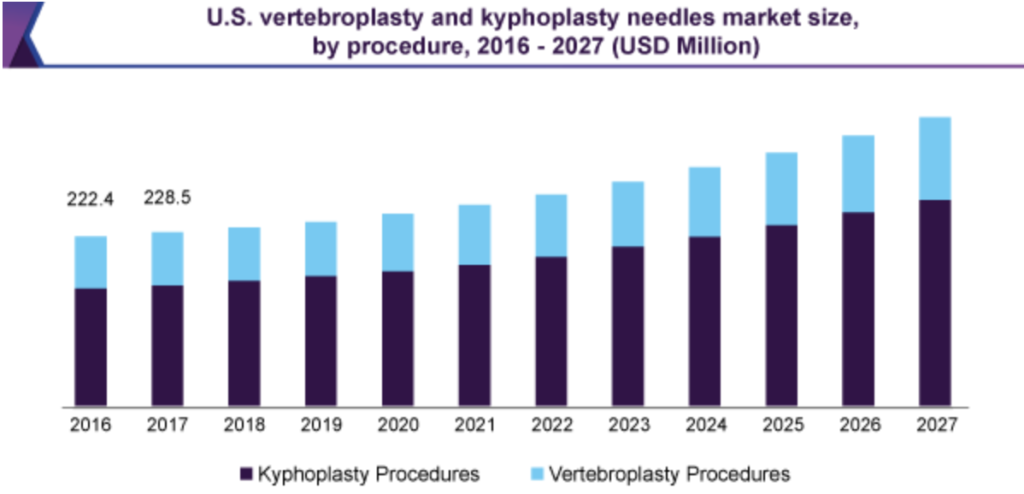
Our device targets the bone biopsy market, along with the clinicians who perform procedures in which they will be used, such as a kyphoplasty, who demand a reliable and consistent method to retrieve sufficiently sized biopsy samples on the first try. The current global $760 million market for bone biopsy needles used for kyphoplasty procedures is expected to grow in the upcoming years, making it one of the primary forces leading the problem we are trying to solve. The market for the needles used during procedures for the treatment of vertebral compression fractures is anticipated to grow at a compound annual growth rate of 5.4% and is projected to reach $3.2 billion between the year 2027 [2].
BioDesign Statement
A medical device that can obtain an adequately sized biopsy sample on the first attempt to prevent increased exposure to potential nerve or muscle damage, inflammation, and infection in the patient when performing a kyphoplasty.
Major Design Requirements
After conducting several voice of customer interviews with neurosurgeons, interventional radiologists, and pathologists we determined our design requirements for the future validation and verification of our biopsy needle.
- Locking Mechanism – this will allow the device to lock and unlock, to limit the amount our blade is exposed
- Screwable End Cap – this will act as a mechanism that allows the sample to be fully encapsulated, and it provides a seal to prevent further sample loss
- Plunger – this additional component will ensure the entire sample is pushed out of the device
- End of Device – this area of our device needs to be capable of piercing through bone, as it will be the first thing that comes in contact with the pedicle
- Sample Size of 2cm in Length (or a volume of 141mm3) – this 2cm length is a set value from clinicians that they have determined is adequate (the volume of the sample was calculated using this length and the current device’s diameter of 3mm)
Prototypes


After several designs and iterations our final prototype, which is a 3x scale model of the device, can be seen above. Our biopsy needle consists of two concentrically fitting tubes, each with an opening that will allow the sample to be guided inward; slots cut into each of the tubes so the blade can slide within it; a blade that is secured on the inner tube and fits in the slot of the outer tube in order to cut/guide the sample; and a screwable end cap that will be removed in order to push the sample out by the plunger. Our device will be inserted, as a whole, into the vertebral body; the inner tube will then rotate, which will expose the blade; the entire system will rotate and that motion will both cut and push the sample into the opening of the tubes; the inner tube will be rotated in the opposite direction closing the system and concealing the blade; then finally the entire system will be removed from the patient.
Testing
Proof of concept testing has been done with our final prototype, specifically with our softer analogs of cottage cheese and porcine liver (representing malignant tissue). It was confirmed that our device was able to collect a length of sample of 1-2cm. We also tested our device on stale bread (representing osteoporotic tissue), but we found that blade bent while rotating, which we believe is due to the material of the blade not being strong enough. Future testing will include further analogs for osteoporotic and healthy tissue, to ensure our device works on all tissue types.
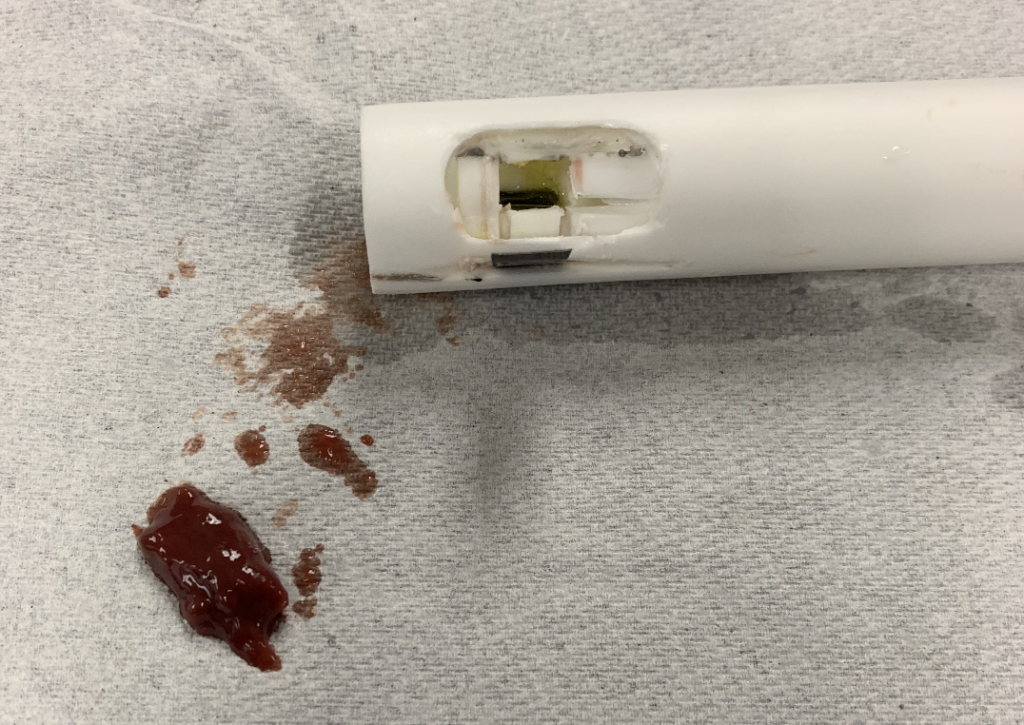
Regulatory Strategy
We have met with FDA Reviewer, Ms. Erin Keegan, to go over our Pre-Submission to the FDA. After gaining her insight, the regulatory pathway we intend to pursue is to market our device through the 510(k) clearance pathway as a Class II device.
Conclusions
Our new bone biopsy needle is an innovative solution for clinicians who struggle to retrieve a sufficiently sized sample, while performing a kyphoplasty. REMA Tech’s promising new device provides a way to collect a bone biopsy sample; and unlike our competitors, our needle can accomplish this on the first try exposing the patient to less risk.
Acknowledgments
REMA Tech would like to thank our close advisors Dr. Greg Gdowski, Martin Gira, Dr. Jonathan Stone, and Matthew Hood for their contributions and constant support in the business, development, and creation of our device!
References
[1] Donnally III CJ, DiPompeo CM, Varacallo M. Vertebral Compression Fractures. [Updated 2020 Mar 25]. In: StatPearls [Internet]. Treasure Island (FL): StatPearls Publishing; 2020 Jan-. https://www.ncbi.nlm.nih.gov/books/NBK448171/
[2] Vertebroplasty And Kyphoplasty Needles Market Size Report, 2027, www.grandviewresearch.com/industry-analysis/vertebroplasty-kyphoplasty-needles-market.



