Problem
A craniotomy is a surgical technique that exposes the brain by removing a section of the bone from the skull. A surgeon makes two or more burr holes and connects them by cutting along the lines to create a bone flap. This gives surgeons access to the dura mater, which is the tough, protective membrane covering the brain. When the bone flap is removed, the dura mater is exposed and it can be gently opened or lifted to gain access to the brain matter beneath it. In older patients, complications can arise due to changes in tissue composition, leading to adhesion of the dura mater to the skull. This adhesion is a challenge during surgery and risks severe damage to the dura mater and underlying brain tissue. This has been a significant concern for neurosurgeons and residents.
Therefore, there is a need for an instrument to make it easier for neurosurgeons to lift the bone flap to allow for safer separation of the dura mater.
Voice of the Customer
“We spend a lot of time making sure the dura is “stripped” from the underside of the skull but this is a blind procedure eg you cant see it while you are doing it.” – Dr. Thomas Mattingly, URMC
“It’s a struggle we all face. You get to pry up the bone flap a little bit so that you can then take your Penfield 3 again and strip once you have more space but it’s like progressive elevation. It comes up a little bit more, you strip a little bit more. And there’s more aggressive people and less aggressive people. Some people just pull really hard and it comes off. Some people spend more time creating the plane correctly.” – Dr. Jonathan Stone, URMC
“The problem really is that if you get under the dura, sometimes you have bridging veins that are very significant in a lot of patients with sinus, and can sometimes run into or even above the dura. Multiple kinds of veins anteriorly that we actually didn’t get to but were above the dura and kind of into that sinus. So, you can have really bad outcomes.” – Dr. Nathaniel Ellens, URMC
Solution
The DuraMate is a fully mechanical device that can be used to elevate the bone flap from the skull during craniotomies and provide access to the dura mater, allowing for a more gentle and easier separation process. It also allows neurosurgeons to have a good tactile field underneath the skull, which is helpful in preventing any risk of damage to the dura mater and the brain underneath.
How to use the DuraMate
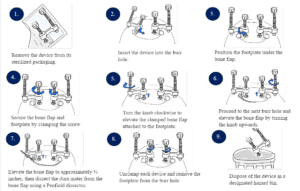
Prototyping
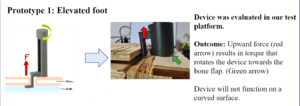



Testing

Market Analysis and Financials
The neurosurgery was valued to be $5.3 billion in 2022 and is projected to reach $8.4 billion by 2032 with a CAGR of 4.8%. More specifically, like the global brain tumor market is expected to reach $31.142 million by the end of 2023, and the aneurysm surgery market is valued at approximately $1.15 billion in 2022. Another market segment examined is the global neurosurgery devices and equipment market. This market was valued at $8.19 billion in 2023 and is expected to reach over $14.84 billion by 2028 with a CAGR of 12.5%. This demand is further amplified by the rising prevalence of brain tumors and aneurysms, particularly among the aging population. Additionally, we assumed approximately 10% of the tumor market will be capturing the New York state hospitals and that is around $3.1142 million.
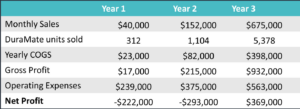
The figure above shows the yearly sales our device would generate. We expect to sell a modest number of products as we are staying local in Rochester area with Strong Memorial Hospital and Highland Hospital initially. In year 2, we plan to expand to upstate NY and hire additional workers to increase our product output. In year 3, we will leverage our connections with hospital coordinators and supply chains to expand and reach 80% of New York state and we will be selling enough products to be profitable.
Team Members

Aveisha Maharaj

Kaveri Salunke

Vincent Mu
Acknowledgements
We would like to thank Dr. Greg Gdowski and Martin Gira for their support in prototyping, planning, and the production of our device through various iterations. Additionally, we would like to thank Dr. Jonathan Stone for his feedback on the prototype and the clinical components of this project, Dr. Joan Adamo for her support through the regulatory process, and Mahllet Beyene for her contributions to planning Design Day.
