Technical Approach
Stage I: Sampling & Characterization
Before testing removal methods, the level of PFAS in the landfill’s leachate had to be characterized. With the help of Brian McGrath, we sampled from the leachate well on site.




Sampling was performed twice, once in February and once in March. These samples were sent to a laboratory in Rochester, ALS Environmental, for a full PFAS analysis. We found that the levels of PFAS were close to those presented in the literature.
Stage II: Prototyping
The PFAS level in the landfill leachate could be quantified at a professional laboratory, but sending many samples to the laboratory was not realistic due to cost restraints. To work around this, we found the limits of the analytical methods we had available and designed our experiments around these limits. This includes high-precision liquid chromatography (HPLC) and scanning electron microscopy (SEM).
We found the HPLC to have a detection limit in the parts per million range. This meant we could test the effectiveness of the GAC and foam methods using highly concentration PFOA solutions and apply our findings to our treatment of the leachate.
Foaming Method
The foaming method developed by Robey et al. uses an aquarium air stone and air pump to bubble air through the liquid solution4. Because of the surfactant properties of PFAS, these molecules tend to form foam which may be scooped off the surface to form concentrated waste.
In order to analyze our results in the HPLC, we first tried this method using PFOA solution. As seen in the video, the solution didn’t foam as expected. Some bubbles were scooped off as they formed, but they settled much faster than those formed in the leachate, suggesting this solution is not representative of the leachate, which foams readily. As such, this prototype experiment was not taken any further.
Granular Activated Carbon Treatment
Activated carbon is a commonly used material for contaminant adsorption. Carbon is activated through oxidation where it is meshed to increase surface area for the adsorption of organic species. We concluded that Filtrasorb400 (F400) would be most suitable for this treatment. Filtrasorb400 was provided by Calgon Carbon 2. Isotherm tests were run to determine feasibility of treatment and a rapid small scale column test (RSSCT) was run to simulate leachate treatment. These two tests were guided by EPA recommendations 3.
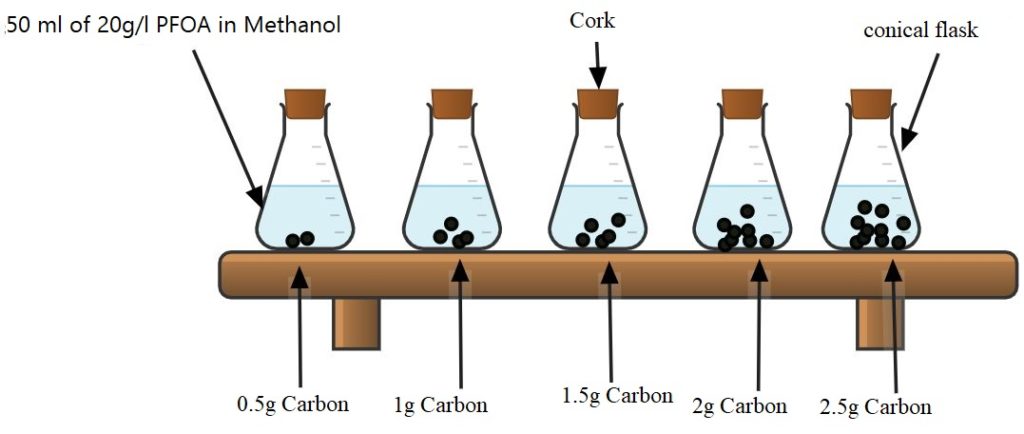
The isotherm test was run with the PFOA prototype solution. The same volume of the PFOA in methanol solution was placed in 5 flasks with varying masses of activated carbon. The flasks were sealed and sampled at different time intervals over a 24 hour period. The samples were tested for PFOA concentration using the HPLC. The results of the isotherm test were used to calculate the adsorption capacity of Filtrasorb 400 activated carbon, with PFOA to be 0.29 g/L PFOA per g carbon. This helped inform how much carbon to use for future experiments based on the concentration of PFOA.
Stage III: Leachate Treatment
The video shows the foaming process for the leachate. Air was pumped at a flowrate of 1.5 mL/min and foam was removed using a scooper until ~75% the original volume remained. These remainders were the first sample sent to the ALS Laboratory.
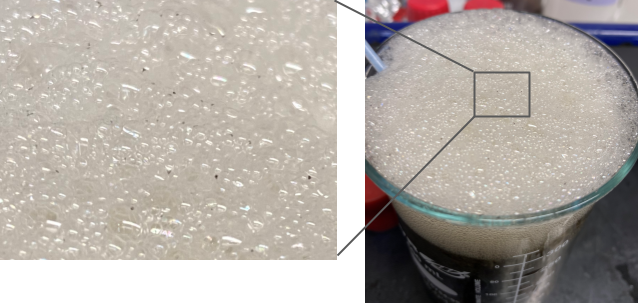
An interesting observation made during the foam process was how solids moved onto the surface, shown below in the image. This is important because treatment of solids was a big unknown in this experiment. If solids containing PFAS may also be removed by this experiment, that would save costs in removing them down the line.
The rapid small scale column test (RSSCT) was carried out by packing a glass column with Filtrasorb 400 activated carbon above a glass wool layer at the bottom, as shown to the right. The leachate was pumped through the column at a rate of 15ml/min. The tubing was fixed to the top of the column to reduce channeling. The treated leachate was run at 50 degrees Fahrenheit to replicate conditions at the landfill.


