Testing and Evaluation
Prototype 1: Pantyliner with Conductive Thread-based sensor
Prototype 2: Hydrogel-based RFID sensor with incorporated Copper Nanoparticles (NPs)
No testing was done on this product as our supervisor informed us that the programming, materials, and expertise required would exceed the amount of time that we have to do the project.
Prototype 3: Copper sheet-based sensor with an NFC IC chip, microcontroller, and NFC-compatible reader.
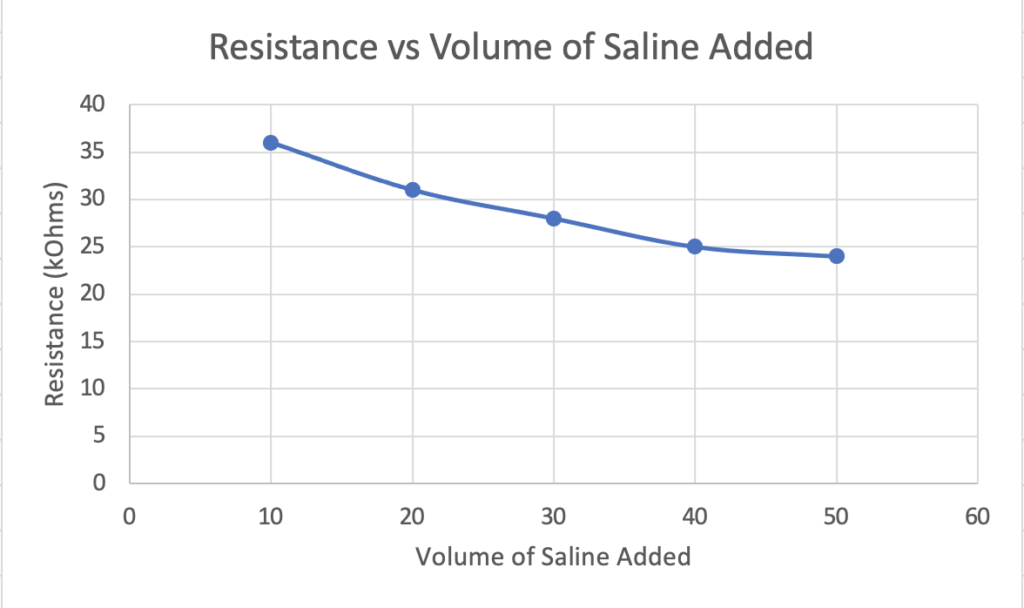
To establish the difference in resistance values in the diaper between dry and wet states, testing was done to ensure these values were different enough to establish a threshold. The setup for our device allowed us to pour a saline solution into the diaper and track the resistance changes.
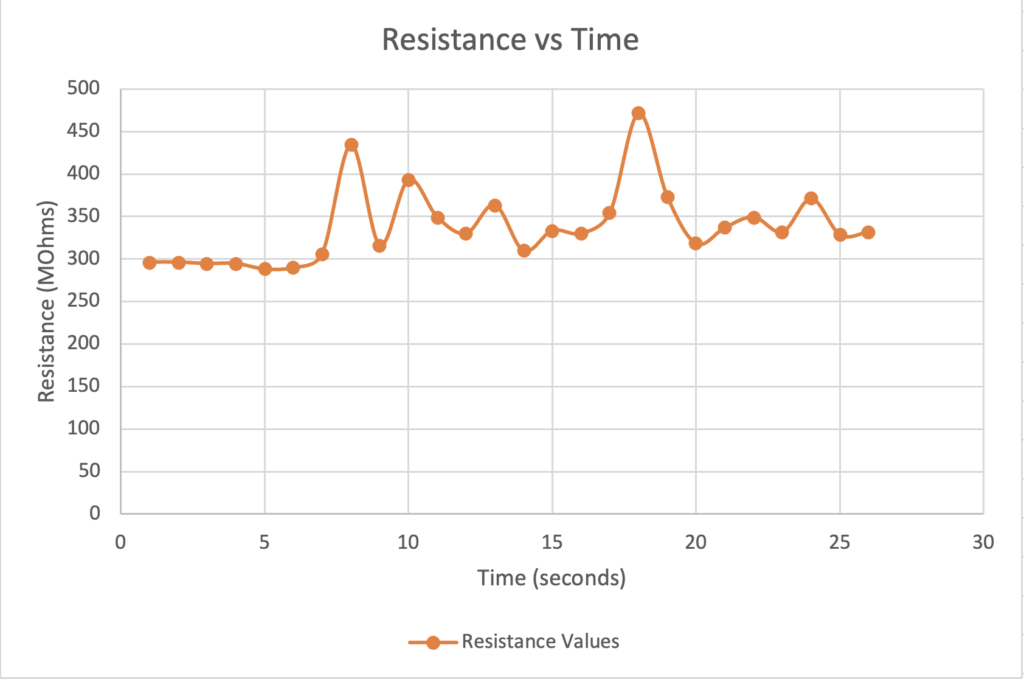
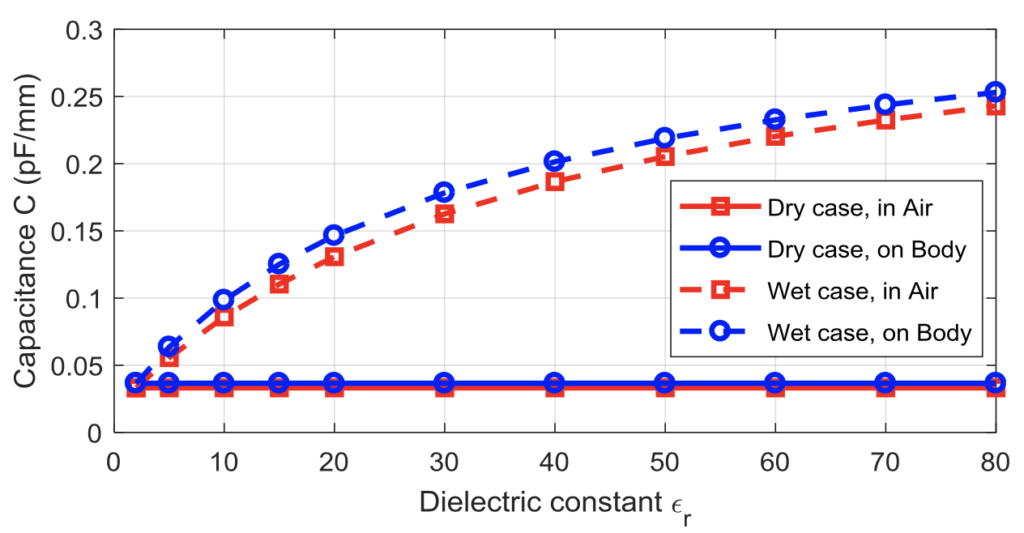
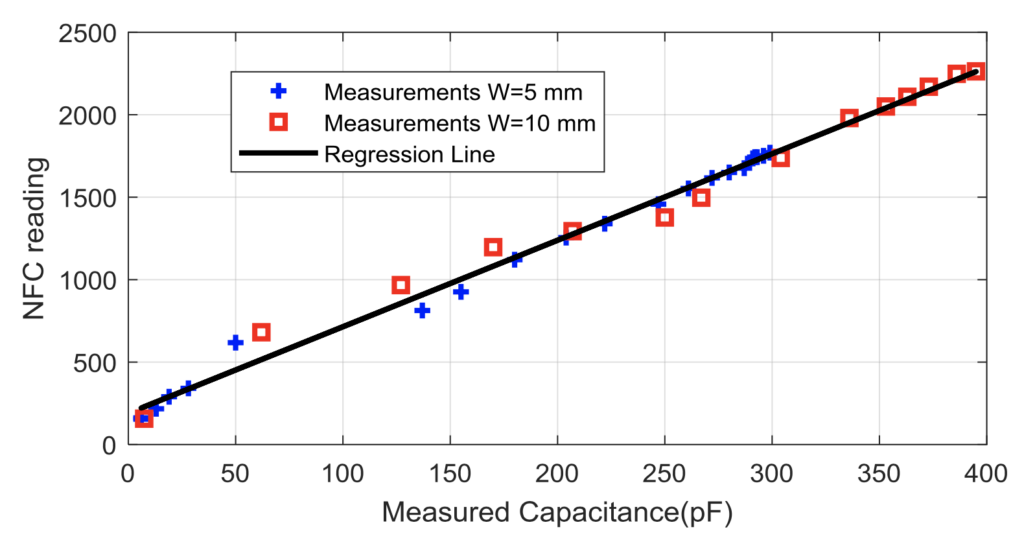
These graphs help illustrate the validity of our prototype that a change in the dielectric constant between the two parallel copper electrodes leads to a change in capacitance upon the introduction of moisture to the diaper (Graph 1) and that a change in capacitance can be measured by the NFC IC chip even when varying the width of the copper electrodes (Graph 2) (Lazaro et al., 2019).
Testing Plan:
This section outlines our planned further testing protocol to ensure device is safe, efficacious, and meets our customer needs and wants specifications.
Biocompatibility Testing
Due to the nature of our product to come into limited duration contact with skin, the FDA requires testing:
- In vivo and in vitro
- Test for material reaction with skin – cytotoxicity, sensitization, and irritation
- Test to ensure radio waves from NFC do not interfere with other essential medical devices (i.e. pacemaker)
Sensor Threshold Testing
- Determine the resistance of the sensor when it is not wet to determine a standard for comparing values.
- Determine the resistance of the sensor when it is wet, as expected from an incontinence event.
- Optimize our sensor for this threshold (difference in resistance between dry vs wet state)
- Test to ensure sensor reliably sends signal when any incontinence event has occurred
Comfort Testing
- Establish that device is comfortable to wear
- Rate comfort on a scale 1-10
- Establish that the device does not cause any pressure sores for the patient.
Communication Testing
- Ensure that the nurse is notified appropriately after an incontinence episode has occurred.
- Ensure that the nurse is notified in a timely manner once the incontinence event has occurred.
