
Problem Statement
Viriotech Biomedical Engineering is designing a cassette for a cost-effective human immunodeficiency virus (HIV) viral load assay. While there has been a steady global decrease in HIV mortality since the turn of the 21st century, sub-Saharan African communities remain disproportionately affected by HIV partly due to limited access to medical resources. Current technology requires patients to wait several days for expensive test results. This wait presents challenges in communities far from health clinics. Our mission is to design a cassette for an HIV viral load assay that allows us to provide quick, more affordable HIV testing for more people in low-resource communities. We are collaborating with Dr. Benjamin Miller and Jeffrey Beard at the University of Rochester Medical Center to develop this device. Our end-users are the healthcare workers in clinics. They will determine a patient’s viral load based on a fluorescence readout. A patient’s blood sample runs through multiple microfluidics paper layers that capture the viral RNA in a detection layer. This cassette incorporates a readout method that uses smartphone technology to determine HIV virion concentration in the blood. Our device has materials that withstand high temperature and humidity environments like in sub-Saharan Africa. Ultimately, this project impacts communities without essential resources in healthcare.
Intellectual Property Disclaimer
Due to IP concerns, some specifications about the device have been omitted.
Background
Aim
Our aim is to design a cassette for a cost-effective and fast HIV viral load assay to increase testing accessibility in areas such as sub-Saharan Africa where HIV rates are highly prevalent.
What is HIV/AIDS?
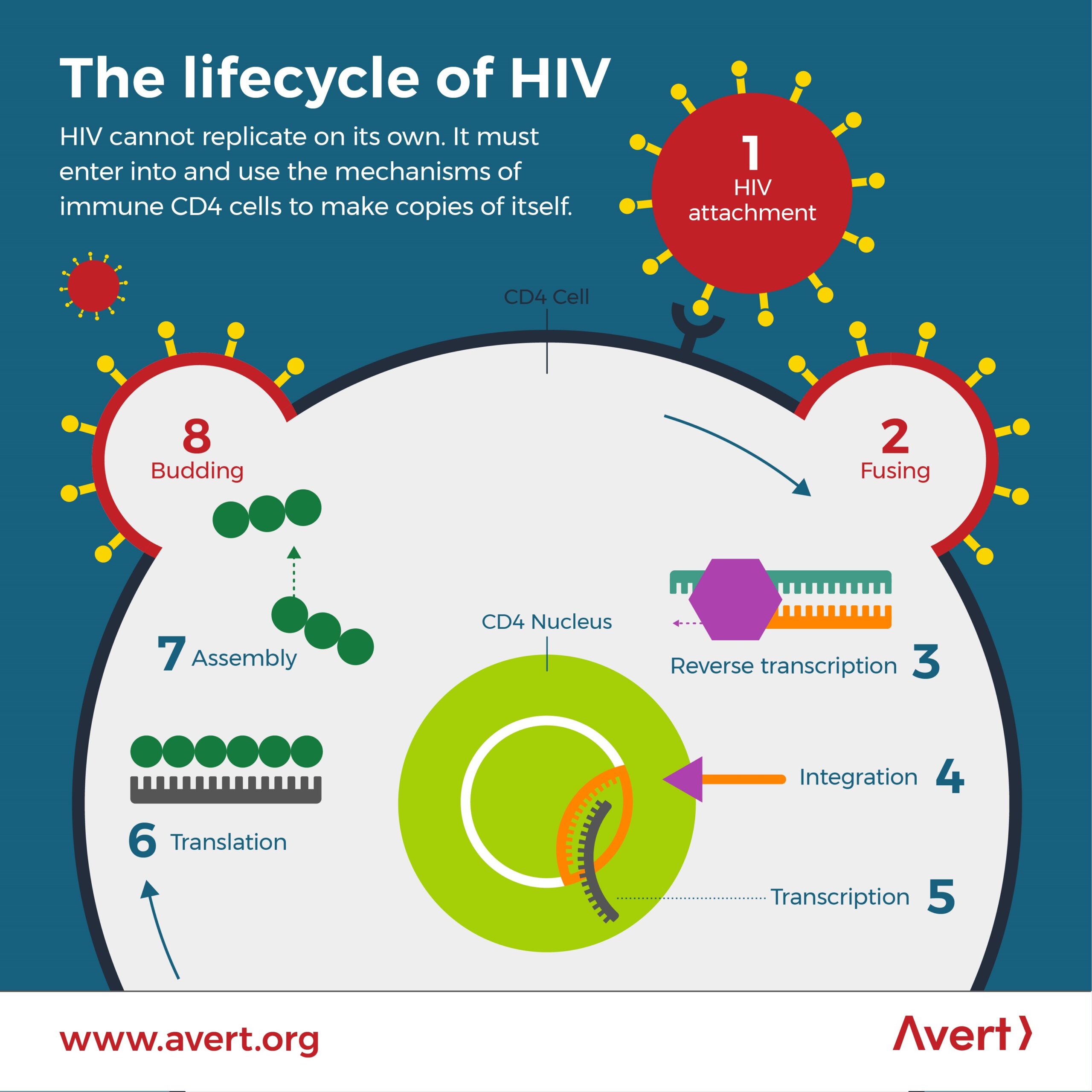
The Human Immunodeficiency Virus (HIV) binds and targets CD4 receptors on T-cells, a type of immune cell that targets foreign invaders in the body. Without treatment, HIV can progress to acquired immunodeficiency syndrome (AIDS) where a patient is completely immunocompromised due to the high viral load of HIV.
Since the first case of HIV in the 1920s, HIV/AIDS was not declared an epidemic until the 1980s. Due to increasing awareness of the manner, there was a major expansion in research in HIV tests and treatments such as antiretroviral therapy (ART). ART prevents the progression of HIV into AIDS and transmission to others.
By 2010, although manageable in most parts of the world due to accessible tests and treatments, new infections remain high in Eastern and Southern Africa.
Below is a schematic of the lifecycle of HIV, from the moment it binds to a CD4 receptor and a coreceptor (CCR5 or CXCR4).
Why is HIV important to address?
Below is a map of HIV prevalence worldwide. Although manageable in most parts of the world due to accessible tests and treatments, 54% of cases are in sub-Saharan Africa.
In 2019, almost 21 million people in Eastern and Southern Africa were living with HIV. This is why our team and customers are focusing on this region.
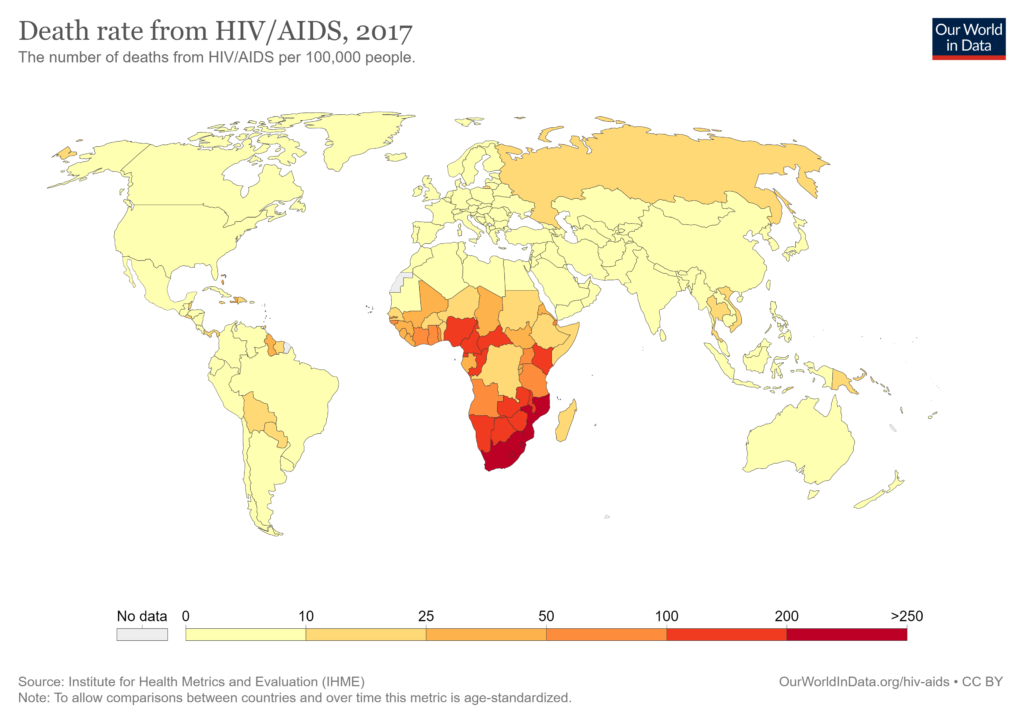
M. Roser and H. Ritchie, “HIV / AIDS,” Our World in Data, 2018.
“Global Statistics”, HIV.gov, 2021
What is a viral load?
Viral load is the gold standard for quantifying the levels of HIV in the blood and therefore determines the success of ART.
By 2021 low to middle-income countries will need an estimated 30 million viral load tests. However, it is not just important to have more people on ART. These patients need to be continuously monitored. The 2013 World Health Organization Guidelines recommend viral load monitoring at six months after treatment initiation, at 12 months, and every 12 months thereafter.
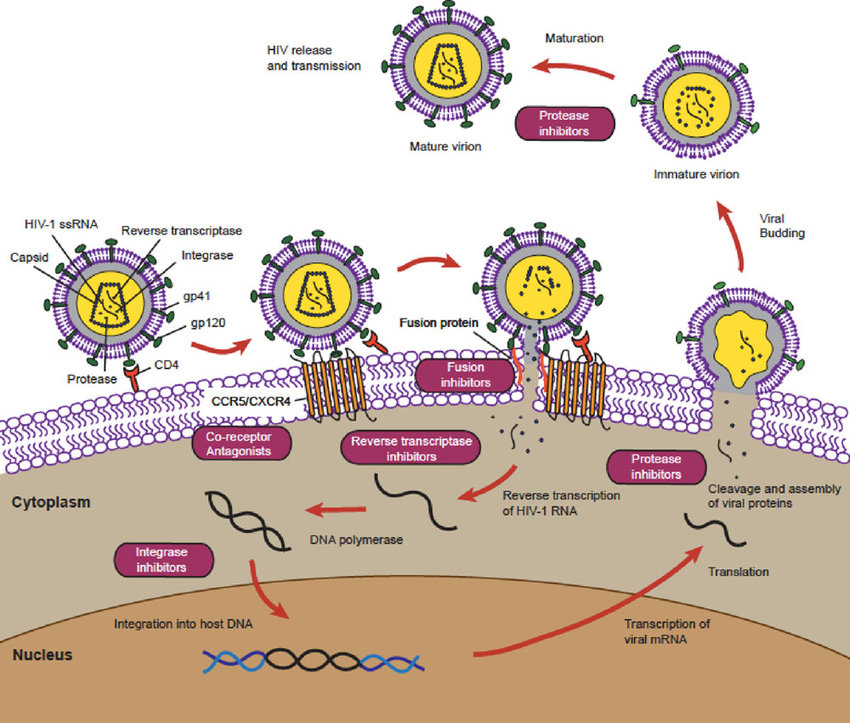
The schematic below shows how antiretroviral drugs (ARVs) target specific steps in the HIV replication cycle.
The Global HIV Market
The global HIV therapeutics market will reach $27 billion by 2023.
The HIV Market encompassing all testing, from diagnostic to viral load assays, exceeded 2.9 billion $ in 2017, out of which 20% is viral load assays.
Below are two popular Viral Load technologies by Cepheid and Abbot.

Why are these technologies not meeting the current need for more, cost-effective viral load assays?
- Require electricity, often not available in the clinics
- Lack of transparency in their prices (apparatus price not listed online)
- One test costs $38-131
Viral load assays with dried blood spots specifically targeted towards middle to low resource communities have prices ranging
- $25-44 without support
- $17-29 with Doctors Without Borders and Unitaid personnel and funding support
“HIV Diagnostics Market Forecasts”, WHO, 2015
P. Drain et al., “Point-of-Care HIV Viral Load Testing: an Essential Tool for a Sustainable Global HIV/AIDS Response”, Clinical Microbiology Reviews, vol. 32, no. 3, 2019.
“How Low Can We Go?”, MSF.org, 2013
