HIV Assay Cassette Design
Customer Scenario
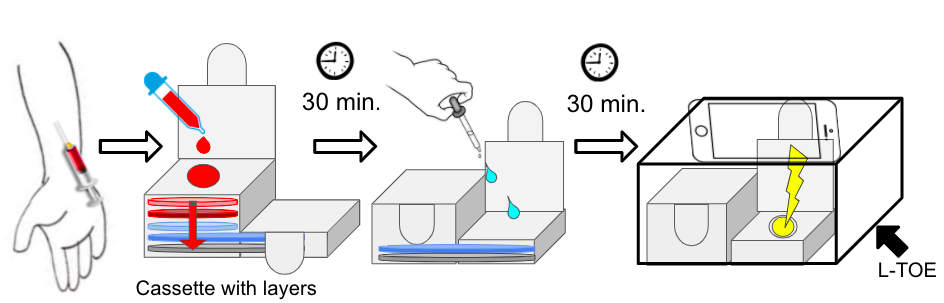
In the clinic, about 1 mL of blood is drawn and taken to the lab for testing. The healthcare worker opens the cassette, inserts the blood sample with the assay reagents, and seals the device to contain infectious materials. Inside the cassette, the blood sample runs through porous layers where viral material can be exposed and analyzed to assess viral load. Within 30 minutes, the test results are available to the patient.
Workflow
The diagram below demonstrates the workflow that healthcare workers will experience in the clinic when using the HIV viral load assay. Please review our customer scenario video to see how this device will work in the clinic.
Device Requirements
- Inexpensive
- Efficient
- Effective
- Stable
- Mass Producible
| Requirements | Metrics |
|---|---|
| Cost (USD) | $10 ± $5 per unit |
| Time before receiving results (min) | 30 – 90 min |
| Blood required (μL) | 750 ± 10 μL |
| Effective diagnosis (%) | 90 – 100% |
| Shelf life (months) | 6+ months |
| Temperature (°C) | 20-50 °C |
| Humidity (%) | 50-90% |
| Number of parts | 2 ± 1 parts |
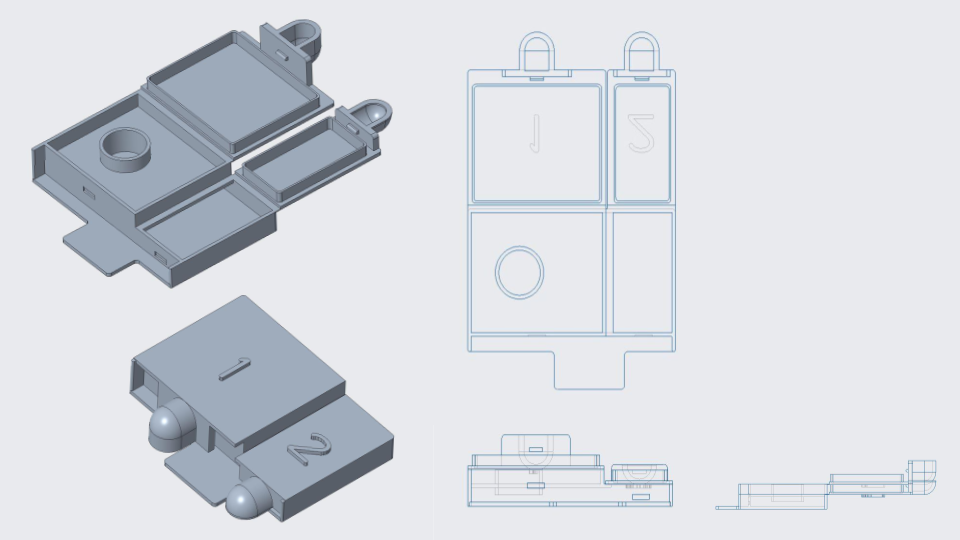
Cassette CAD & 3D Printed Model
As shown in the figures, the cassette features two lids in different sizes with a blood inlet. In clinical settings, medical workers should collect about 1 mL of blood from patients and apply it to the inlet through lid #1. After 30 minutes, an image is taken with a phone through lid #2 for the result.
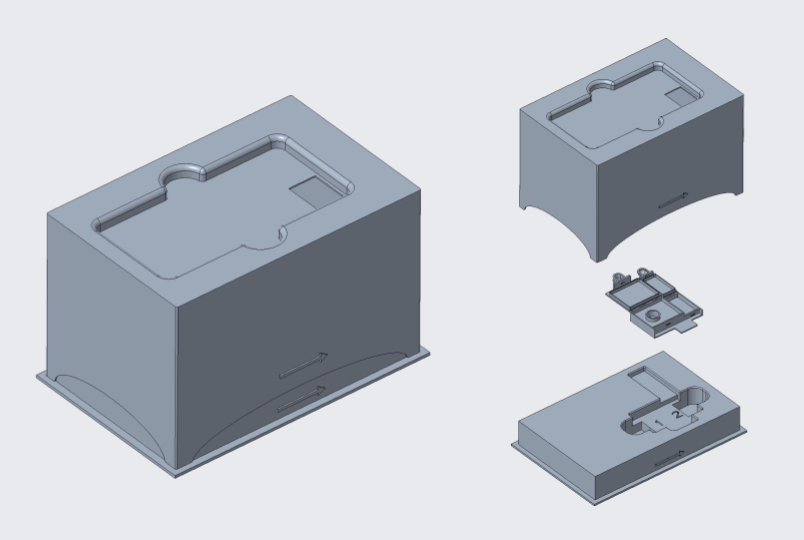
L-TOE CAD & 3D Printed Model
The figures demonstrate the Light-Tight Optical Enclosure (L-TOE) that will be used to take the image for the result. At the bottom of the device, there is a sunken part in the shape of the cassette with lid #2 open. The cassette can be slide in through the gap on the side of the top half of L-TOE. The phone can be secured in place on top of the device. In the future, the L-TOE will be manufacturing using cardboard.
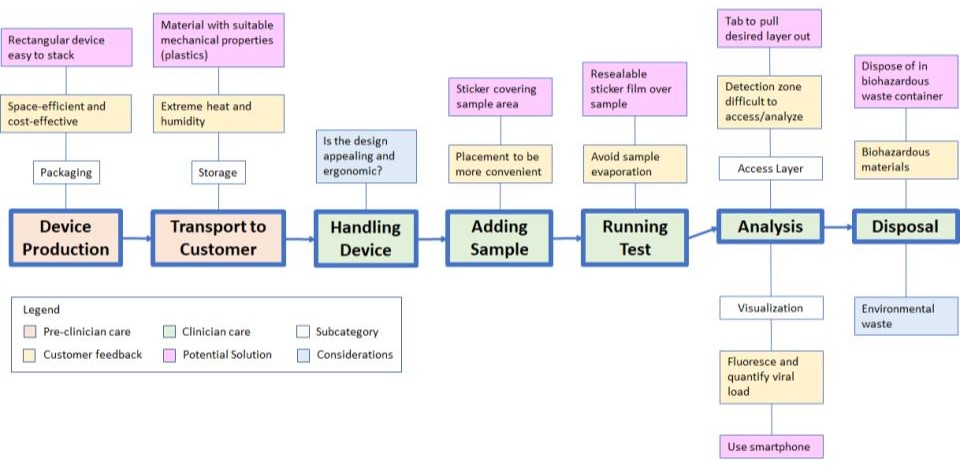
Life Cycle